HIV-1 Reverse Transcriptase Still Remains a New Drug Target: Structure, Function, Classical Inhibitors, and New Inhibitors with Innovative Mechanisms of Actions
- PMID: 22778958
- PMCID: PMC3388302
- DOI: 10.1155/2012/586401
HIV-1 Reverse Transcriptase Still Remains a New Drug Target: Structure, Function, Classical Inhibitors, and New Inhibitors with Innovative Mechanisms of Actions
Abstract
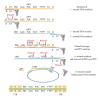
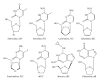
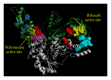
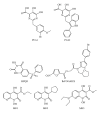
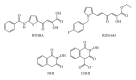
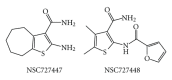
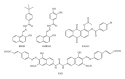
During the retrotranscription process, characteristic of all retroviruses, the viral ssRNA genome is converted into integration-competent dsDNA. This process is accomplished by the virus-coded reverse transcriptase (RT) protein, which is a primary target in the current treatments for HIV-1 infection. In particular, in the approved therapeutic regimens two classes of drugs target RT, namely, nucleoside RT inhibitors (NRTIs) and nonnucleoside RT inhibitors (NNRTIs). Both classes inhibit the RT-associated polymerase activity: the NRTIs compete with the natural dNTP substrate and act as chain terminators, while the NNRTIs bind to an allosteric pocket and inhibit polymerization noncompetitively. In addition to these two classes, other RT inhibitors (RTIs) that target RT by distinct mechanisms have been identified and are currently under development. These include translocation-defective RTIs, delayed chain terminators RTIs, lethal mutagenesis RTIs, dinucleotide tetraphosphates, nucleotide-competing RTIs, pyrophosphate analogs, RT-associated RNase H function inhibitors, and dual activities inhibitors. This paper describes the HIV-1 RT function and molecular structure, illustrates the currently approved RTIs, and focuses on the mechanisms of action of the newer classes of RTIs.
Figures
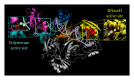

Similar articles
-
HIV-1 RT Inhibitors with a Novel Mechanism of Action: NNRTIs that Compete with the Nucleotide Substrate.Viruses. 2010 Apr;2(4):880-899. doi: 10.3390/v2040880. Epub 2010 Mar 30. Viruses. 2010. PMID: 21994659 Free PMC article.
-
Cutting into the Substrate Dominance: Pharmacophore and Structure-Based Approaches toward Inhibiting Human Immunodeficiency Virus Reverse Transcriptase-Associated Ribonuclease H.Acc Chem Res. 2020 Jan 21;53(1):218-230. doi: 10.1021/acs.accounts.9b00450. Epub 2019 Dec 27. Acc Chem Res. 2020. PMID: 31880912 Free PMC article. Review.
-
Susceptibility of Human Endogenous Retrovirus Type K to Reverse Transcriptase Inhibitors.J Virol. 2017 Nov 14;91(23):e01309-17. doi: 10.1128/JVI.01309-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28931682 Free PMC article.
-
Identification of mechanistically distinct inhibitors of HIV-1 reverse transcriptase through fragment screening.Proc Natl Acad Sci U S A. 2015 Jun 2;112(22):6979-84. doi: 10.1073/pnas.1423900112. Epub 2015 May 18. Proc Natl Acad Sci U S A. 2015. PMID: 26038551 Free PMC article.
-
Nonnucleoside inhibitors of HIV-1 reverse transcriptase: from the biology of reverse transcription to molecular design.Curr Top Med Chem. 2003;3(13):1496-511. doi: 10.2174/1568026033451754. Curr Top Med Chem. 2003. PMID: 14529523 Review.
Cited by
-
Determining chemical reactivity driving biological activity from SMILES transformations: the bonding mechanism of anti-HIV pyrimidines.Molecules. 2013 Jul 30;18(8):9061-116. doi: 10.3390/molecules18089061. Molecules. 2013. PMID: 23903183 Free PMC article.
-
New targets for HIV drug discovery.Drug Discov Today. 2019 May;24(5):1139-1147. doi: 10.1016/j.drudis.2019.03.013. Epub 2019 Mar 15. Drug Discov Today. 2019. PMID: 30885676 Free PMC article. Review.
-
Cytotoxic and HIV-1 enzyme inhibitory activities of Red Sea marine organisms.BMC Complement Altern Med. 2014 Feb 25;14:77. doi: 10.1186/1472-6882-14-77. BMC Complement Altern Med. 2014. PMID: 24568567 Free PMC article.
-
Docking-based 3D-QSAR and pharmacophore studies on diarylpyrimidines as non-nucleoside inhibitors of HIV-1 reverse transcriptase.Mol Divers. 2019 Feb;23(1):107-121. doi: 10.1007/s11030-018-9860-1. Epub 2018 Jul 26. Mol Divers. 2019. PMID: 30051344
-
Novel 2-(Diphenylmethylidene) Malonic Acid Derivatives as Anti-HIV Agents: Molecular Modeling, Synthesis and Biological Evaluation.Iran J Pharm Res. 2021 Dec 14;21(1):e123827. doi: 10.5812/ijpr.123827. eCollection 2022 Dec. Iran J Pharm Res. 2021. PMID: 35765501 Free PMC article.
References
-
- Barré-Sinoussi F, Chermann JC, Rey F, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220(4599):868–871. - PubMed
-
- Broder S, Gallo RC. A pathogenic retrovirus (HTLV-III) linked to AIDS. New England Journal of Medicine. 1984;311(20):1292–1297. - PubMed
-
- Mehellou Y, De Clercq E. Twenty-six years of anti-HIV drug discovery: where do we stand and where do we go? Journal of Medicinal Chemistry. 2010;53(2):521–538. - PubMed
-
- Ratner L, Haseltine W, Patarca R. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985;313(6000):277–284. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
