Role of the pituitary–adrenal axis in granulocyte-colony stimulating factor-induced neuroprotection against hypoxia–ischemia in neonatal rats
- PMID: 22779090
- PMCID: PMC3606051
- DOI: 10.1016/j.nbd.2012.03.021
Role of the pituitary–adrenal axis in granulocyte-colony stimulating factor-induced neuroprotection against hypoxia–ischemia in neonatal rats
Abstract
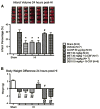
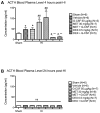
Several reports indicate that the activity of the hypothalamic–pituitary–adrenal axis (HPA) is increased after a brain insult and that its down-regulation can improve detrimental outcomes associated with ischemic brain injuries.Granulocyte-colony stimulating factor (G-CSF) is a neuroprotective drug shown in the naïve rat to regulate hormones of the HPA axis. In this study we investigate whether G-CSF confers its neuroprotective properties by influencing the HPA response after neonatal hypoxia–ischemia (HI). Following the Rice–Vannucci model, seven day old rats (P7)were subjected to unilateral carotid ligation followed by 2.5 h of hypoxia. To test our hypothesis,metyrapone was administered to inhibit the release of rodent specific glucocorticoid, corticosterone, at the adrenal level. Dexamethasone, a synthetic glucocorticoid, was administered to agonize the effects of corticosterone.Our results show that both G-CSF and metyrapone significantly reduced infarct volume while dexamethasone treatment did not reduce infarct size even when combined with G-CSF. The protective effects of G-CSF do not include blood brain barrier preservation as suggested by the brain edema results. G-CSF did not affect the pituitary released adrenocorticotropic hormone (ACTH) levels in the blood plasma at 4 h, but suppressed the increase of corticosterone in the blood. The administration of G-CSF and metyrapone increased weight gain, and significantly reduced the Bax/Bcl-2 ratio in the brain while dexamethasone reversed the effects of G-CSF. The combination of G-CSF and metyrapone significantly decreased caspase-3 protein levels in the brain, and the effect was antagonized by dexamethasone.We report that G-CSF is neuroprotective in neonatal HI by reducing infarct volume, by suppressing the HI-induced increase of the Bax/Bcl-2 ratio, and by decreasing corticosterone in the blood. Metyrapone was able to confer similar neuroprotection as G-CSF while dexamethasone reversed the effects of G-CSF. In conclusion, we show that decreasing HPA axis activity is neuroprotective after neonatal HI, which can be conferred by administering G-CSF.
Figures




References
-
- Allen C, Kendall JW. Maturation of the circadian rhythm of plasma corticosterone in the rat. Endocrinology. 1967 May;80(5):926–930. - PubMed
-
- Betz AL, Coester HC. Effect of steroids on edema and sodium uptake of the brain during focal ischemia in rats. Stroke. 1990 Aug;21(8):1199–1204. - PubMed
-
- Butt TK, Farooqui R, Khan MA. Risk factors for hypoxic ischemic encephalopathy in children. J Coll Physicians Surg Pak. 2008 Jul;18(7):428–432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
