Design and synthesis of neuroprotective methylthiazoles and modification as NO-chimeras for neurodegenerative therapy
- PMID: 22779770
- PMCID: PMC3680370
- DOI: 10.1021/jm300353r
Design and synthesis of neuroprotective methylthiazoles and modification as NO-chimeras for neurodegenerative therapy
Abstract
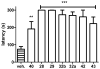
Learning and memory deficits in Alzheimer's disease (AD) result from synaptic failure and neuronal loss, the latter caused in part by excitotoxicity and oxidative stress. A therapeutic approach is described that uses NO-chimeras directed at restoration of both synaptic function and neuroprotection. 4-Methylthiazole (MZ) derivatives were synthesized, based upon a lead neuroprotective pharmacophore acting in part by GABA(A) receptor potentiation. MZ derivatives were assayed for protection of primary neurons against oxygen-glucose deprivation and excitotoxicity. Selected neuroprotective derivatives were incorporated into NO-chimera prodrugs, coined nomethiazoles. To provide proof of concept for the nomethiazole drug class, selected examples were assayed for restoration of synaptic function in hippocampal slices from AD-transgenic mice, reversal of cognitive deficits, and brain bioavailability of the prodrug and its neuroprotective MZ metabolite. Taken together, the assay data suggest that these chimeric nomethiazoles may be of use in treatment of multiple components of neurodegenerative disorders, such as AD.
Conflict of interest statement
Conflict of Interest Disclosure: GRJT has a consulting relationship with sGC Pharma, an entity that currently licenses intellectual property associated with nomethiazoles from the University of Illinois.
Figures




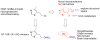


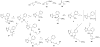
References
-
- Wimo A, Winblad B, Jonsson L. The worldwide societal costs of dementia: Estimates for 2009. Alzheimers Dement. 2010;6:98–103. - PubMed
-
- Cummings JL. Alzheimer’s disease. N Engl J Med. 2004;351:56–67. - PubMed
-
- Selkoe DJ. Resolving controversies on the path to Alzheimer’s therapeutics. Nat Med. 2011;17:1060–1065. - PubMed
-
- Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer’s disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–716. - PubMed
-
- Citron M. Alzheimer’s disease: strategies for disease modification. Nat Rev Drug Discov. 2010;9:387–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

