Cationic antimicrobial peptides disrupt the Streptococcus pyogenes ExPortal
- PMID: 22780862
- PMCID: PMC3646575
- DOI: 10.1111/j.1365-2958.2012.08163.x
Cationic antimicrobial peptides disrupt the Streptococcus pyogenes ExPortal
Abstract
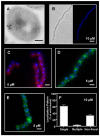
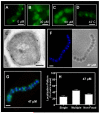
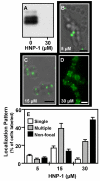
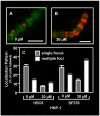
Although they possess a well-characterized ability to porate the bacterial membrane, emerging research suggests that cationic antimicrobial peptides (CAPs) can influence pathogen behaviour at levels that are sublethal. In this study, we investigated the interaction of polymyxin B and human neutrophil peptide (HNP-1) with the human pathogen Streptococcus pyogenes. At sublethal concentrations, these CAPs preferentially targeted the ExPortal, a unique microdomain of the S. pyogenes membrane, specialized for protein secretion and processing. A consequence of this interaction was the disruption of ExPortal organization and a redistribution of ExPortal components into the peripheral membrane. Redistribution was associated with inhibition of secretion of certain toxins, including the SpeB cysteine protease and the streptolysin O (SLO) cytolysin, but not SIC, a protein that protects S. pyogenes from CAPs. These data suggest a novel function for CAPs in targeting the ExPortal and interfering with secretion of factors required for infection and survival. This mechanism may prove valuable for the design of new types of antimicrobial agents to combat the emergence of antibiotic-resistant pathogens.
© 2012 Blackwell Publishing Ltd.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

