Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma
- PMID: 22780919
- PMCID: PMC3472555
- DOI: 10.1089/hum.2012.041
Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma
Abstract
No curative treatment exists for glioblastoma, with median survival times of less than 2 years from diagnosis. As an approach to develop immune-based therapies for glioblastoma, we sought to target antigens expressed in glioma stem cells (GSCs). GSCs have multiple properties that make them significantly more representative of glioma tumors than established glioma cell lines. Epidermal growth factor receptor variant III (EGFRvIII) is the result of a novel tumor-specific gene rearrangement that produces a unique protein expressed in approximately 30% of gliomas, and is an ideal target for immunotherapy. Using PCR primers spanning the EGFRvIII-specific deletion, we found that this tumor-specific gene is expressed in three of three GCS lines. Based on the sequence information of seven EGFRvIII-specific monoclonal antibodies (mAbs), we assembled chimeric antigen receptors (CARs) and evaluated the ability of CAR-engineered T cells to recognize EGFRvIII. Three of these anti-EGFRvIII CAR-engineered T cells produced the effector cytokine, interferon-γ, and lysed antigen-expressing target cells. We concentrated development on a CAR produced from human mAb 139, which specifically recognized GSC lines and glioma cell lines expressing mutant EGFRvIII, but not wild-type EGFR and did not recognize any normal human cell tested. Using the 139-based CAR, T cells from glioblastoma patients could be genetically engineered to recognize EGFRvIII-expressing tumors and could be expanded ex vivo to large numbers, and maintained their antitumor activity. Based on these observations, a γ-retroviral vector expressing this EGFRvIII CAR was produced for clinical application.
Trial registration: ClinicalTrials.gov NCT01454596.
Figures
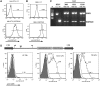



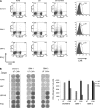

References
-
- Bao S. Wu Q. McLendon R.E., et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006a;444:756–760. - PubMed
-
- Bao S. Wu Q. Sathornsumetee S., et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006b;66:7843–7848. - PubMed
-
- Boockvar J.A. Kapitonov D. Kapoor G., et al. Constitutive EGFR signaling confers a motile phenotype to neural stem cells. Mol. Cell. Neurosci. 2003;24:1116–1130. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

