Metformin and phenethyl isothiocyanate combined treatment in vitro is cytotoxic to ovarian cancer cultures
- PMID: 22781119
- PMCID: PMC3439343
- DOI: 10.1186/1757-2215-5-19
Metformin and phenethyl isothiocyanate combined treatment in vitro is cytotoxic to ovarian cancer cultures
Abstract
Background: High mortality rates in ovarian cancer are largely a result of resistance to currently used chemotherapies. Expanding therapies with a variety of drugs has the potential to reduce this high mortality rate. Metformin and phenethyl isothiocyanate (PEITC) are both potentially useful in ovarian cancer, and they are particularly attractive because of their safety.
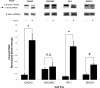
Methods: Cell proliferation of each drug and drug combination was evaluated by hemacytometry with Trypan blue exclusion or Sytox green staining for cell death. Levels of total and cleaved PARP were measured by Western blot. General cellular and mitochondrial reactive oxygen species were measured by flow cytometry and live cell confocal microscopy with the fluorescent dyes dihydroethidine and MitoSOX.
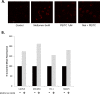
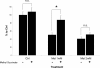
Results: Individually, metformin and PEITC each show inhibition of cell growth in multiple ovarian cancer cell lines. Alone, PEITC was also able to induce apoptosis, whereas metformin was primarily growth inhibitory. Both total cellular and mitochondrial reactive oxygen species were increased when treated with either metformin or PEITC. The growth inhibitory effects of metformin were reversed by methyl succinate supplementation, suggesting complex I plays a role in metformin's anti-cancer mechanism. PEITC's anti-cancer effect was reversed by N-acetyl-cysteine supplementation, suggesting PEITC relies on reactive oxygen species generation to induce apoptosis. Metformin and PEITC together showed a synergistic effect on ovarian cancer cell lines, including the cisplatin resistant A2780cis.
Conclusions: Here we show that when used in combination, these drugs are effective in both slowing cancer cell growth and killing ovarian cancer cells in vitro. Furthermore, the combination of these drugs remains effective in cisplatin resistant cell lines. Novel combinations such as metformin and PEITC show promise in expanding ovarian cancer therapies and overcoming the high incidence of cisplatin resistant cancers.
Figures

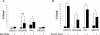
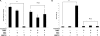
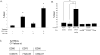
Similar articles
-
Phenethyl Isothiocyanate Induces Apoptotic Cell Death Through the Mitochondria-dependent Pathway in Gefitinib-resistant NCI-H460 Human Lung Cancer Cells In Vitro.Anticancer Res. 2018 Apr;38(4):2137-2147. doi: 10.21873/anticanres.12454. Anticancer Res. 2018. PMID: 29599332
-
Phenethyl Isothiocyanate Enhances the Cytotoxic Effects of PARP Inhibitors in High-Grade Serous Ovarian Cancer Cells.Front Oncol. 2022 Jan 26;11:812264. doi: 10.3389/fonc.2021.812264. eCollection 2021. Front Oncol. 2022. PMID: 35155204 Free PMC article.
-
Cisplatin in combination with Phenethyl Isothiocyanate (PEITC), a potential new therapeutic strategy for malignant pleural mesothelioma.Oncotarget. 2014 Nov 30;5(22):11641-52. doi: 10.18632/oncotarget.2604. Oncotarget. 2014. PMID: 25361002 Free PMC article.
-
Pharmacokinetics and pharmacodynamics of phenethyl isothiocyanate: implications in breast cancer prevention.AAPS J. 2014 Jul;16(4):705-13. doi: 10.1208/s12248-014-9610-y. Epub 2014 May 13. AAPS J. 2014. PMID: 24821055 Free PMC article. Review.
-
Phenethyl isothiocyanate: a comprehensive review of anti-cancer mechanisms.Biochim Biophys Acta. 2014 Dec;1846(2):405-24. doi: 10.1016/j.bbcan.2014.08.003. Epub 2014 Aug 23. Biochim Biophys Acta. 2014. PMID: 25152445 Free PMC article. Review.
Cited by
-
Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers.Molecules. 2018 Nov 15;23(11):2983. doi: 10.3390/molecules23112983. Molecules. 2018. PMID: 30445746 Free PMC article. Review.
-
New molecules and old drugs as emerging approaches to selectively target human glioblastoma cancer stem cells.Biomed Res Int. 2014;2014:126586. doi: 10.1155/2014/126586. Epub 2014 Jan 2. Biomed Res Int. 2014. PMID: 24527434 Free PMC article. Review.
-
Safe and targeted anticancer therapy for ovarian cancer using a novel class of curcumin analogs.J Ovarian Res. 2013 May 11;6(1):35. doi: 10.1186/1757-2215-6-35. J Ovarian Res. 2013. PMID: 23663277 Free PMC article.
-
Metformin improves the sensitivity of ovarian cancer cells to chemotherapeutic agents.Oncol Lett. 2019 Sep;18(3):2404-2411. doi: 10.3892/ol.2019.10564. Epub 2019 Jul 4. Oncol Lett. 2019. PMID: 31402943 Free PMC article.
-
Metformin against cancer stem cells through the modulation of energy metabolism: special considerations on ovarian cancer.Biomed Res Int. 2014;2014:132702. doi: 10.1155/2014/132702. Epub 2014 Jun 24. Biomed Res Int. 2014. PMID: 25050322 Free PMC article. Review.
References
-
- Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, Reding DJ, Greenlee RT, Yokochi LA, Kessel B. et al.Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305:2295–2303. doi: 10.1001/jama.2011.766. - DOI - PubMed
-
- Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

