Increased intracranial pressure after diffuse traumatic brain injury exacerbates neuronal somatic membrane poration but not axonal injury: evidence for primary intracranial pressure-induced neuronal perturbation
- PMID: 22781336
- PMCID: PMC3463883
- DOI: 10.1038/jcbfm.2012.95
Increased intracranial pressure after diffuse traumatic brain injury exacerbates neuronal somatic membrane poration but not axonal injury: evidence for primary intracranial pressure-induced neuronal perturbation
Abstract
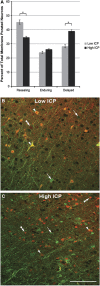
Increased intracranial pressure (ICP) associated with traumatic brain injury (TBI) is linked to increased morbidity. Although our understanding of the pathobiology of TBI has expanded, questions remain regarding the specific neuronal somatic and axonal damaging consequences of elevated ICP, independent of its impact on cerebral perfusion pressure (CPP). To investigate this, Fischer rats were subjected to moderate TBI. Measurements of ICP revealed two distinct responses to injury. One population exhibited transient increases in ICP that returned to baseline levels acutely, while the other displayed persistent ICP elevation (>20 mm Hg). Utilizing these populations, the effect of elevated ICP on neuronal pathology associated with diffuse TBI was analyzed at 6 hours after TBI. No difference in axonal injury was observed, however, rats exhibiting persistently elevated ICP postinjury revealed a doubling of neurons with chronic membrane poration compared with rats exhibiting only transient increases in ICP. Elevated postinjury ICP was not associated with a concurrent increase in DNA damage; however, traditional histological assessments did reveal increased neuronal damage, potentially associated with redistribution of cathepsin-B from the lysosomal compartment into the cytosol. These findings indicate that persistently increased ICP, without deleterious alteration of CPP, exacerbates neuronal plasmalemmal perturbation that could precipitate persistent neuronal impairment and ultimate neuronal death.
Figures






Similar articles
-
Moderately elevated intracranial pressure after diffuse traumatic brain injury is associated with exacerbated neuronal pathology and behavioral morbidity in the rat.J Cereb Blood Flow Metab. 2014 Oct;34(10):1628-36. doi: 10.1038/jcbfm.2014.122. Epub 2014 Jul 16. J Cereb Blood Flow Metab. 2014. PMID: 25027309 Free PMC article.
-
Intracranial pressure elevations in diffuse axonal injury: association with nonhemorrhagic MR lesions in central mesencephalic structures.J Neurosurg. 2018 Sep 14;131(2):604-611. doi: 10.3171/2018.4.JNS18185. Print 2019 Aug 1. J Neurosurg. 2018. PMID: 30215559
-
Cathepsin B Relocalization in Late Membrane Disrupted Neurons Following Diffuse Brain Injury in Rats.ASN Neuro. 2022 Jan-Dec;14:17590914221099112. doi: 10.1177/17590914221099112. ASN Neuro. 2022. PMID: 35503242 Free PMC article.
-
Indomethacin: a review of its cerebral blood flow effects and potential use for controlling intracranial pressure in traumatic brain injury patients.Neurol Res. 1999 Jul;21(5):491-9. Neurol Res. 1999. PMID: 10439431 Review.
-
Cellular and subcellular change evoked by diffuse traumatic brain injury: a complex web of change extending far beyond focal damage.Prog Brain Res. 2007;161:43-59. doi: 10.1016/S0079-6123(06)61004-2. Prog Brain Res. 2007. PMID: 17618969 Review.
Cited by
-
Cathepsin B in neurodegeneration of Alzheimer's disease, traumatic brain injury, and related brain disorders.Biochim Biophys Acta Proteins Proteom. 2020 Aug;1868(8):140428. doi: 10.1016/j.bbapap.2020.140428. Epub 2020 Apr 17. Biochim Biophys Acta Proteins Proteom. 2020. PMID: 32305689 Free PMC article. Review.
-
Emerging therapies in traumatic brain injury.Semin Neurol. 2015 Feb;35(1):83-100. doi: 10.1055/s-0035-1544237. Epub 2015 Feb 25. Semin Neurol. 2015. PMID: 25714870 Free PMC article. Review.
-
Cooling Strategies Targeting Trauma.Ther Hypothermia Temp Manag. 2014 Mar 1;4(1):3-7. doi: 10.1089/ther.2014.1502. Ther Hypothermia Temp Manag. 2014. PMID: 24660098 Free PMC article. No abstract available.
-
Traumatically injured astrocytes release a proteomic signature modulated by STAT3-dependent cell survival.Glia. 2016 May;64(5):668-94. doi: 10.1002/glia.22953. Epub 2015 Dec 19. Glia. 2016. PMID: 26683444 Free PMC article.
-
Intranasal Administration of the Antisecretory Peptide AF-16 Reduces Edema and Improves Cognitive Function Following Diffuse Traumatic Brain Injury in the Rat.Front Neurol. 2017 Feb 14;8:39. doi: 10.3389/fneur.2017.00039. eCollection 2017. Front Neurol. 2017. PMID: 28261150 Free PMC article.
References
-
- Bramlett HM, Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab. 2004;24:133–150. - PubMed
-
- Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, Schouten J, Shutter L, Timmons SD, Ullman JS, Videtta W, Wilberger JE, Wright DW.2007aGuidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds J Neurotrauma 24(Suppl 1S59–S64.(Information also online at https://www.braintrauma.org/coma-guidelines/) - PubMed
-
- Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, Schouten J, Shutter L, Timmons SD, Ullman JS, Videtta W, Wilberger JE, Wright DW.2007bGuidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring J Neurotrauma 24(Suppl 1S37–S44.(Information also online at https://www.braintrauma.org/coma-guidelines/) - PubMed
-
- Choo AM, Liu J, Lam CK, Dvorak M, Tetzlaff W, Oxland TR. Contusion, dislocation, and distraction: primary hemorrhage and membrane permeability in distinct mechanisms of spinal cord injury. J Neurosurg Spine. 2007;6:255–266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

