Oncolytic virotherapy
- PMID: 22781695
- PMCID: PMC3888062
- DOI: 10.1038/nbt.2287
Oncolytic virotherapy
Abstract
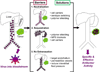
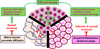
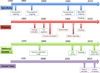
Oncolytic virotherapy is an emerging treatment modality that uses replication-competent viruses to destroy cancers. Recent advances include preclinical proof of feasibility for a single-shot virotherapy cure, identification of drugs that accelerate intratumoral virus propagation, strategies to maximize the immunotherapeutic action of oncolytic viruses and clinical confirmation of a critical viremic threshold for vascular delivery and intratumoral virus replication. The primary clinical milestone has been completion of accrual in a phase 3 trial of intratumoral herpes simplex virus therapy using talimogene laherparepvec for metastatic melanoma. Key challenges for the field are to select 'winners' from a burgeoning number of oncolytic platforms and engineered derivatives, to transiently suppress but then unleash the power of the immune system to maximize both virus spread and anticancer immunity, to develop more meaningful preclinical virotherapy models and to manufacture viruses with orders-of-magnitude higher yields than is currently possible.
Conflict of interest statement
Dr. Bell is the Chief Scientific Officer of Jennerex Biotherapeutics.
Figures
References
-
- DM K, CE S, P P. In: Fields Virology. Edn. Fourth Edition. DM K, et al., editors. Vol. I. Philadelphia: Lippincott Williams & Wilkins; 2001.
-
- Dorer DE, Nettelbeck DM. Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv Drug Deliv Rev. 2009;61:554–571. - PubMed
-
- Naik S, Russell SJ. Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin Biol Ther. 2009;9:1163–1176. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 CA136547/CA/NCI NIH HHS/United States
- R01 CA168719/CA/NCI NIH HHS/United States
- CA129966/CA/NCI NIH HHS/United States
- CA100634/CA/NCI NIH HHS/United States
- R01 CA129193/CA/NCI NIH HHS/United States
- CA136547/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- CA136393/CA/NCI NIH HHS/United States
- CA118488/CA/NCI NIH HHS/United States
- R01 CA100634/CA/NCI NIH HHS/United States
- P50 CA136393/CA/NCI NIH HHS/United States
- R01 CA129966/CA/NCI NIH HHS/United States
- CA129193/CA/NCI NIH HHS/United States
- R01 CA118488/CA/NCI NIH HHS/United States
- N01 CA015083/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

