The role of the cilium in normal and abnormal cell cycles: emphasis on renal cystic pathologies
- PMID: 22782110
- PMCID: PMC3657316
- DOI: 10.1007/s00018-012-1052-z
The role of the cilium in normal and abnormal cell cycles: emphasis on renal cystic pathologies
Abstract
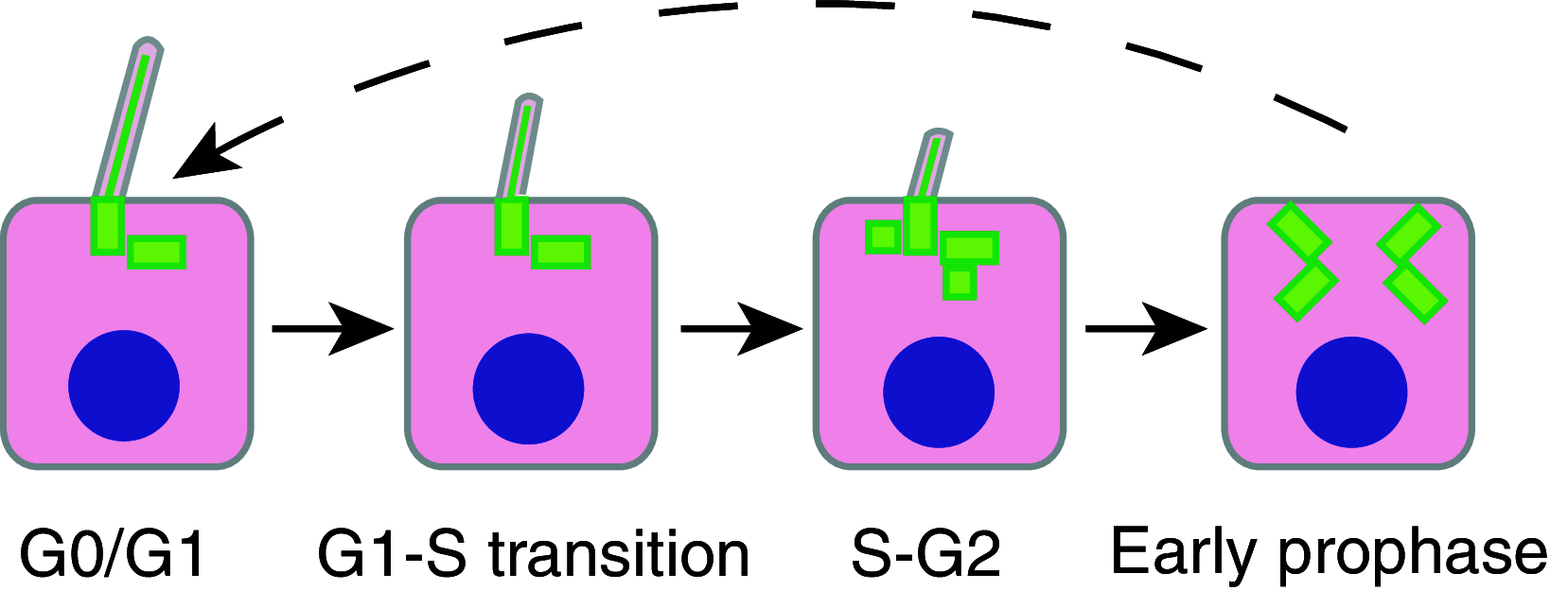
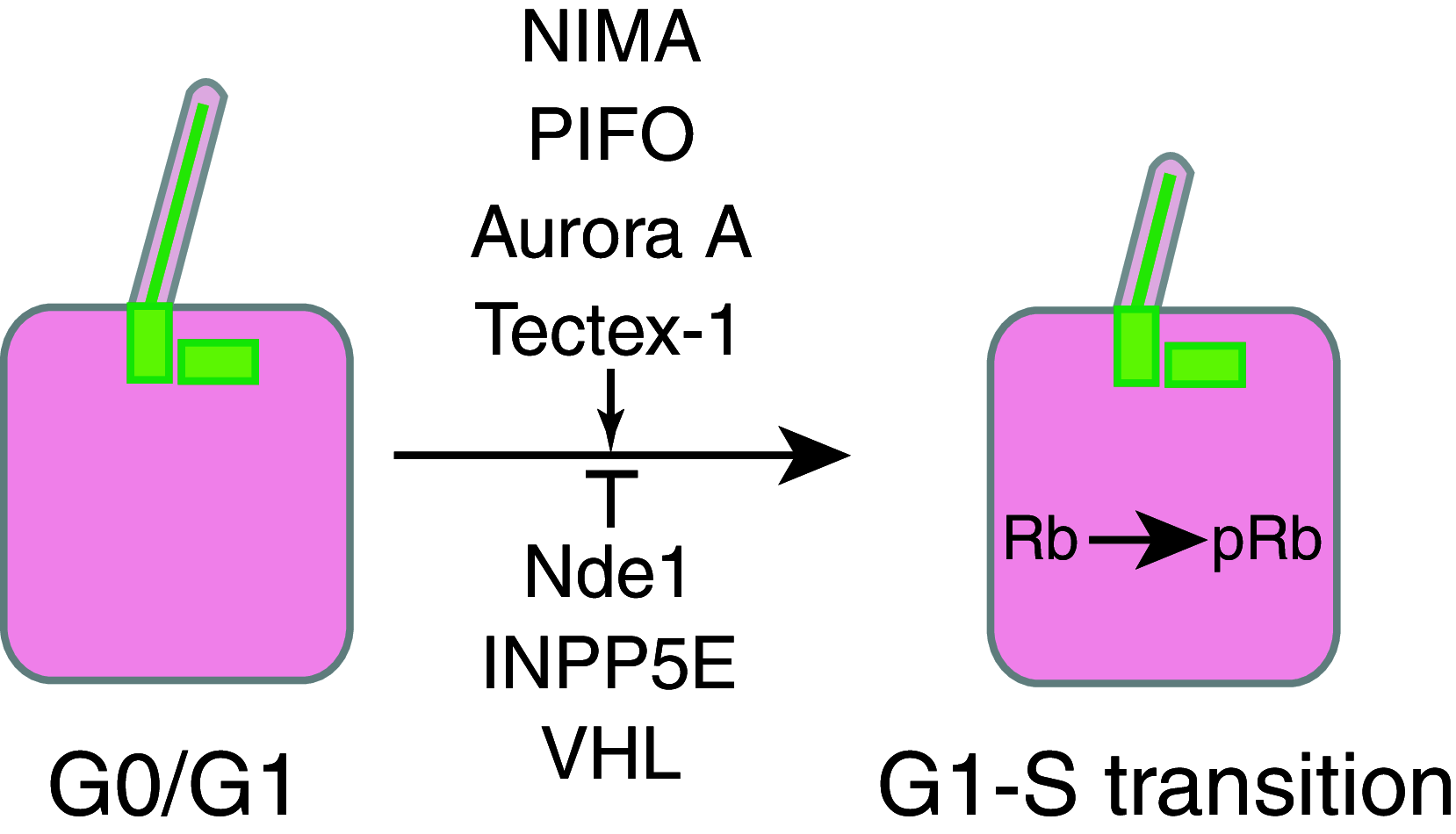
The primary cilium protrudes from the cell surface and acts as a sensor for chemical and mechanical growth cues, with receptors for a number of growth factors (PDGFα, Hedgehog, Wnt, Notch) concentrated within the ciliary membrane. In normal tissues, the cilium assembles after cells exit mitosis and is resorbed as part of cell cycle re-entry. Although regulation of the cilium by cell cycle transitions has been appreciated for over 100 years, only recently have data emerged to indicate the cilium also exerts influence on the cell cycle. The resorption/protrusion cycle, regulated by proteins including Aurora-A, VHL, and GSK-3β, influences cell responsiveness to growth cues involving cilia-linked receptors; further, resorption liberates the ciliary basal body to differentiate into the centrosome, which performs discrete functions in S-, G2-, and M-phase. Besides these roles, the cilium provides a positional cue that regulates polarity of cell division, and thus directs cells towards fates of differentiation versus proliferation. In this review, we summarize the specific mechanisms mediating the cilia-cell cycle dialog. We then emphasize the examples of polycystic kidney disease (PKD), nephronopthisis (NPHP), and VHL-linked renal cysts as cases in which defects of ciliary function influence disease pathology, and may also condition response to treatment.
Figures


Similar articles
-
Primary cilia and the cell cycle.Methods Cell Biol. 2009;94:137-60. doi: 10.1016/S0091-679X(08)94007-3. Epub 2009 Dec 23. Methods Cell Biol. 2009. PMID: 20362089 Free PMC article.
-
pVHL and GSK3beta are components of a primary cilium-maintenance signalling network.Nat Cell Biol. 2007 May;9(5):588-95. doi: 10.1038/ncb1579. Epub 2007 Apr 22. Nat Cell Biol. 2007. PMID: 17450132
-
Formation of primary cilia in the renal epithelium is regulated by the von Hippel-Lindau tumor suppressor protein.J Am Soc Nephrol. 2006 Jul;17(7):1801-6. doi: 10.1681/ASN.2006020181. Epub 2006 Jun 14. J Am Soc Nephrol. 2006. PMID: 16775032
-
Cilium, centrosome and cell cycle regulation in polycystic kidney disease.Biochim Biophys Acta. 2011 Oct;1812(10):1263-71. doi: 10.1016/j.bbadis.2011.02.008. Epub 2011 Mar 2. Biochim Biophys Acta. 2011. PMID: 21376807 Free PMC article. Review.
-
All along the watchtower: is the cilium a tumor suppressor organelle?Biochim Biophys Acta. 2008 Dec;1786(2):114-25. doi: 10.1016/j.bbcan.2008.02.002. Epub 2008 Feb 26. Biochim Biophys Acta. 2008. PMID: 18343234 Review.
Cited by
-
Loss of Pkd1 limits susceptibility to colitis and colorectal cancer.Oncogenesis. 2023 Aug 5;12(1):40. doi: 10.1038/s41389-023-00486-y. Oncogenesis. 2023. PMID: 37542051 Free PMC article.
-
Calcium signaling in polycystic kidney disease- cell death and survival.Cell Calcium. 2023 Jun;112:102733. doi: 10.1016/j.ceca.2023.102733. Epub 2023 Mar 31. Cell Calcium. 2023. PMID: 37023534 Free PMC article. Review.
-
Anticystogenic activity of a small molecule PAK4 inhibitor may be a novel treatment for autosomal dominant polycystic kidney disease.Kidney Int. 2017 Oct;92(4):922-933. doi: 10.1016/j.kint.2017.03.031. Epub 2017 May 23. Kidney Int. 2017. PMID: 28545714 Free PMC article.
-
The exocyst gene Sec10 regulates renal epithelial monolayer homeostasis and apoptotic sensitivity.Am J Physiol Cell Physiol. 2015 Aug 1;309(3):C190-201. doi: 10.1152/ajpcell.00011.2015. Epub 2015 Jun 3. Am J Physiol Cell Physiol. 2015. PMID: 26040895 Free PMC article.
-
Intraflagellar Transport Proteins as Regulators of Primary Cilia Length.Front Cell Dev Biol. 2021 May 19;9:661350. doi: 10.3389/fcell.2021.661350. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34095126 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

