Efficacy and safety of capecitabine plus cisplatin in Japanese patients with advanced or metastatic gastric cancer: subset analyses of the AVAGAST study and the ToGA study
- PMID: 22782463
- PMCID: PMC3627028
- DOI: 10.1007/s10120-012-0167-0
Efficacy and safety of capecitabine plus cisplatin in Japanese patients with advanced or metastatic gastric cancer: subset analyses of the AVAGAST study and the ToGA study
Erratum in
- Gastric Cancer. 2013 Apr;16(2):183-4
Abstract
Background: Capecitabine plus cisplatin (XP) is recognized as one of the global standard first-line chemotherapy regimens for patients with metastatic gastric cancer (mGC). Recent multinational phase III trials in mGC have been conducted with XP as the control arm, although no data on XP in Japanese patients with mGC have been published to date. The AVAGAST (XP ± bevacizumab in mGC) and ToGA (XP ± trastuzumab in human epidermal growth factor receptor 2 [HER2]-positive mGC) studies were the first two global studies including Japanese mGC patients. The aim of this analysis was to investigate the efficacy and safety of XP in Japanese mGC patients, using AVAGAST and ToGA subgroup data.
Methods: Efficacy and safety analyses were carried out in Japanese patients with mGC receiving XP alone, based on results from the AVAGAST and ToGA studies. There were differences in the target populations between the two studies; for example, the ToGA study limited patients to those with HER2-positive tumors; therefore, efficacy was evaluated separately.
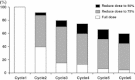
Results: Ninety-four Japanese patients in the AVAGAST study and 50 in the ToGA study received XP alone. Median overall and progression-free survivals were 14.2 and 5.7 months, respectively, in the AVAGAST study, and 17.7 and 5.6 months, respectively, in the ToGA study. Overall response rates were 49.2 % in the AVAGAST and 58.5 % in the ToGA study. Adverse events were generally mild; the most common grade 3/4 events were neutropenia, anemia, anorexia, and nausea.
Conclusions: XP is effective and well tolerated in Japanese patients with mGC, and could be one of the standard regimens for the first-line treatment in this cohort.
Figures

References
-
- Ferlay J, Bray F, Parkin DM, Pisani P, editors. Gobocan 2000: cancer incidence and mortality worldwide (IARC Cancer Bases No. 5). Lyon: IARC Press; 2001.
-
- Cancer Statistics in Japan. Foundation for promotion of cancer research. 2010. http://ganjoho.ncc.go.jp/public/statistics/backnumber/2010_en.html. Accessed 19 January 2011.
-
- Okines AF, Norman AR, McCloud P, Kang YK, Cunningham D. Meta-analysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol. 2009;20:1529–1534. doi: 10.1093/annonc/mdp047. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

