N-glycosylation of enhanced aromatic sequons to increase glycoprotein stability
- PMID: 22782562
- PMCID: PMC3539202
- DOI: 10.1002/bip.22030
N-glycosylation of enhanced aromatic sequons to increase glycoprotein stability
Abstract
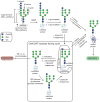
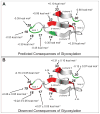
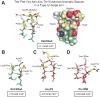
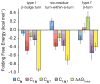
N-glycosylation can increase the rate of protein folding, enhance thermodynamic stability, and slow protein unfolding; however, the molecular basis for these effects is incompletely understood. Without clear engineering guidelines, attempts to use N-glycosylation as an approach for stabilizing proteins have resulted in unpredictable energetic consequences. Here, we review the recent development of three "enhanced aromatic sequons," which appear to facilitate stabilizing native-state interactions between Phe, Asn-GlcNAc and Thr when placed in an appropriate reverse turn context. It has proven to be straightforward to engineer a stabilizing enhanced aromatic sequon into glycosylation-naïve proteins that have not evolved to optimize specific protein-carbohydrate interactions. Incorporating these enhanced aromatic sequons into appropriate reverse turn types within proteins should enhance the well-known pharmacokinetic benefits of N-glycosylation-based stabilization by lowering the population of protease-susceptible unfolded and aggregation-prone misfolded states, thereby making such proteins more useful in research and pharmaceutical applications.
Copyright © 2012 Wiley Periodicals, Inc.
Figures




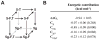


References
-
- Kornfeld R, Kornfeld S. Annu Rev Biochem. 1985;54:631–664. - PubMed
-
- Yan A, Lennarz WJ. J Biol Chem. 2005;280:3121–3124. - PubMed
-
- Kelleher DJ, Gilmore R. Glycobiology. 2006;16:47R–62R. - PubMed
-
- Helenius A, Aebi M. Science. 2001;291:2364–2369. - PubMed
-
- Molinari M. Nat Chem Biol. 2007;3:313–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

