Lysophosphatidic acid induces migration of human lung-resident mesenchymal stem cells through the β-catenin pathway
- PMID: 22782863
- PMCID: PMC3645326
- DOI: 10.1002/stem.1171
Lysophosphatidic acid induces migration of human lung-resident mesenchymal stem cells through the β-catenin pathway
Abstract
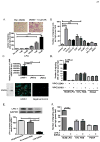
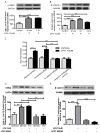
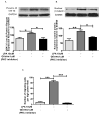
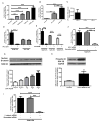
Mesenchymal stem cells (MSCs) have been demonstrated to reside in human adult organs. However, mechanisms of migration of these endogenous MSCs within their tissue of origin are not well understood. Here, we investigate migration of human adult lung-resident (LR) mesenchymal progenitor cells. We demonstrate that bioactive lipid lysophosphatidic acid (LPA) plays a principal role in the migration of human LR-MSCs through a signaling pathway involving LPA1-induced β-catenin activation. LR-MSCs isolated from human lung allografts and lungs of patients with scleroderma demonstrated a robust migratory response to LPA in vitro. Furthermore, LPA levels correlated with LR-MSC numbers in bronchoalveolar lavage (BAL), providing demonstration of the in vivo activity of LPA in human adult lungs. Migration of LR-MSCs was mediated via LPA1 receptor ligation and LPA1 silencing significantly abrogated the migratory response of LR-MSCs to LPA as well as human BAL. LPA treatment of LR-MSCs induced protein kinase C-mediated glycogen synthase kinase-3β phosphorylation, with resulting cytoplasmic accumulation and nuclear translocation of β-catenin. TCF/LEF dual luciferase gene reporter assay demonstrated a significant increase in transcriptional activity after LPA treatment. LR-MSC migration and increase in reporter gene activity in the presence of LPA were abolished by transfection with β-catenin small interfering RNA demonstrating that β-catenin is critical in mediating LPA-induced LR-MSC migration. These data delineate a novel signaling pathway through which ligation of a G protein-coupled receptor by a biologically relevant lipid mediator induces migration of human tissue-resident mesenchymal progenitors.
Copyright © 2012 AlphaMed Press.
Figures

References
-
- Belperio JA, Weigt SS, Fishbein MC, et al. Chronic lung allograft rejection: mechanisms and therapy. Proc Am Thorac Soc. 2009;6:108–121. - PubMed
-
- Hoyles RK, Derrett-Smith EC, Khan K, et al. An essential role for resident fibroblasts in experimental lung fibrosis is defined by lineage-specific deletion of high-affinity type II transforming growth factor beta receptor. Am J Respir Crit Care Med. 2011;183:249–261. - PubMed
-
- Bruno S, Bussolati B, Grange C, et al. Isolation and Characterization of Resident Mesenchymal Stem Cells in Human Glomeruli. Stem cells and development. 2009 - PubMed

