Targeting serous epithelial ovarian cancer with designer zinc finger transcription factors
- PMID: 22782891
- PMCID: PMC3436144
- DOI: 10.1074/jbc.M112.360768
Targeting serous epithelial ovarian cancer with designer zinc finger transcription factors
Abstract
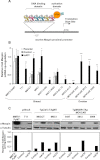
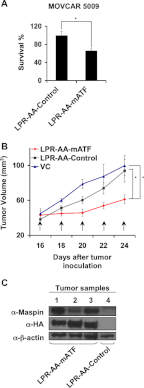
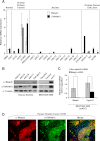
Ovarian cancer is the leading cause of death among gynecological malignancies. It is detected at late stages when the disease is spread through the abdominal cavity in a condition known as peritoneal carcinomatosis. Thus, there is an urgent need to develop novel therapeutic interventions to target advanced stages of ovarian cancer. Mammary serine protease inhibitor (Maspin) represents an important metastasis suppressor initially identified in breast cancer. Herein we have generated a sequence-specific zinc finger artificial transcription factor (ATF) to up-regulate the Maspin promoter in aggressive ovarian cancer cell lines and to interrogate the therapeutic potential of Maspin in ovarian cancer. We found that although Maspin was expressed in some primary ovarian tumors, the promoter was epigenetically silenced in cell lines derived from ascites. Transduction of the ATF in MOVCAR 5009 cells derived from ascitic cultures of a TgMISIIR-TAg mouse model of ovarian cancer resulted in tumor cell growth inhibition, impaired cell invasion, and severe disruption of actin cytoskeleton. Systemic delivery of lipid-protamine-RNA nanoparticles encapsulating a chemically modified ATF mRNA resulted in inhibition of ovarian cancer cell growth in nude mice accompanied with Maspin re-expression in the treated tumors. Gene expression microarrays of ATF-transduced cells revealed an exceptional specificity for the Maspin promoter. These analyses identified novel targets co-regulated with Maspin in human short-term cultures derived from ascites, such as TSPAN12, that could mediate the anti-metastatic phenotype of the ATF. Our work outlined the first targeted, non-viral delivery of ATFs into tumors with potential clinical applications for metastatic ovarian cancers.
Figures




References
-
- Jemal A., Bray F., Center M. M., Ferlay J., Ward E., Forman D. (2011) Global cancer statistics. CA Cancer J. Clin. 61, 69–90 - PubMed
-
- Havrilesky L., Darcy M., Hamdan H., Priore R. L., Leon J., Bell J., Berchuck A. (2003) Prognostic significance of p53 mutation and p53 overexpression in advanced epithelial ovarian cancer. A gynecologic oncology group study. J. Clin. Oncol. 21, 3814–3825 - PubMed
-
- Risch H. A., McLaughlin J. R., Cole D. E., Rosen B., Bradley L., Kwan E., Jack E., Vesprini D. J., Kuperstein G., Abrahamson J. L., Fan I., Wong B., Narod S. A. (2001) Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am. J. Hum. Genet. 68, 700–710 - PMC - PubMed
-
- Liu J., Matulonis U. A. (2010) New advances in ovarian cancer. Oncology 24, 721–728 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

