Cathepsin B is secreted apically from Xenopus 2F3 cells and cleaves the epithelial sodium channel (ENaC) to increase its activity
- PMID: 22782900
- PMCID: PMC3436264
- DOI: 10.1074/jbc.M111.338574
Cathepsin B is secreted apically from Xenopus 2F3 cells and cleaves the epithelial sodium channel (ENaC) to increase its activity
Abstract
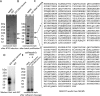
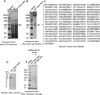
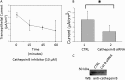
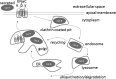
The epithelial sodium channel (ENaC) plays an important role in regulating sodium balance, extracellular volume, and blood pressure. Evidence suggests the α and γ subunits of ENaC are cleaved during assembly before they are inserted into the apical membranes of epithelial cells, and maximal activity of ENaC depends on cleavage of the extracellular loops of α and γ subunits. Here, we report that Xenopus 2F3 cells apically express the cysteine protease cathepsin B, as indicated by two-dimensional gel electrophoresis and mass spectrometry analysis. Recombinant GST ENaC α, β, and γ subunit fusion proteins were expressed in Escherichia coli and then purified and recovered from bacterial inclusion bodies. In vitro cleavage studies revealed the full-length ENaC α subunit fusion protein was cleaved by active cathepsin B but not the full-length β or γ subunit fusion proteins. Both single channel patch clamp studies and short circuit current experiments show ENaC activity decreases with the application of a cathepsin B inhibitor directly onto the apical side of 2F3 cells. We suggest a role for the proteolytic cleavage of ENaC by cathepsin B, and we suggest two possible mechanisms by which cathepsin B could regulate ENaC. Cathepsin B may cleave ENaC extracellularly after being secreted or intracellularly, while ENaC is present in the Golgi or in recycling endosomes.
Figures




References
-
- Lu C., Pribanic S., Debonneville A., Jiang C., Rotin D. (2007) The PY motif of ENaC, mutated in Liddle syndrome, regulates channel internalization, sorting, and mobilization from subapical pool. Traffic 8, 1246–1264 - PubMed
-
- Konstas A. A., Mavrelos D., Korbmacher C. (2000) Conservation of pH sensitivity in the epithelial sodium channel (ENaC) with Liddle's syndrome mutation. Pflugers Arch. 441, 341–350 - PubMed
-
- Prince L. S., Welsh M. J. (1999) Effect of subunit composition and Liddle's syndrome mutations on biosynthesis of ENaC. Am. J. Physiol. 276, C1346–C1351 - PubMed
-
- Schild L. (1996) The ENaC channel as the primary determinant of two human diseases. Liddle syndrome and pseudohypoaldosteronism. Nephrologie 17, 395–400 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

