Funny Current and Cardiac Rhythm: Insights from HCN Knockout and Transgenic Mouse Models
- PMID: 22783204
- PMCID: PMC3387723
- DOI: 10.3389/fphys.2012.00240
Funny Current and Cardiac Rhythm: Insights from HCN Knockout and Transgenic Mouse Models
Abstract
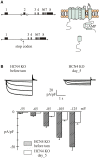
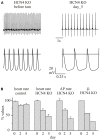
In the adult animal the sinoatrial node (SAN) rhythmically generates a depolarizing wave that propagates to the rest of the heart. However, the SAN is more than a simple clock; it is a clock that adjusts its pace according to the metabolic requirements of the organism. The Hyperpolarization-activated Cyclic Nucleotide-gated channels (HCN1-4) are the structural component of the funny (I(f)) channels; in the SAN the I(f) current is the main driving electrical force of the diastolic depolarization and the HCN4 is the most abundant isoform. The generation of HCN KO and transgenic mouse models has advanced the understanding of the role of these channels in cardiac excitability. The HCN4 KO models that were first developed allowed either global or cardiac-specific constitutive ablation of HCN4 channels, and resulted in embryonic lethality. A further progress was made with the development of three separate inducible HCN4 KO models; in one model KO was induced globally in the entire organism, in a second, ablation occurred only in HCN4-expressing cells, and finally in a third model KO was confined to cardiac cells. Unexpectedly, the three models yielded different results; similarities and differences among these models will be presented and discussed. The functional effects of HCN2 and HCN3 knockout models and transgenic HCN4 mouse models will also be discussed. In conclusion, HCN KO/transgenic models have allowed to evaluate the functional role of the I(f) currents in intact animals as well as in single SAN cells isolated from the same animals. This opportunity is therefore unique since it allows (1) to verify the contribution of specific HCN isoforms to cardiac activity in intact animals, and (2) to compare these results to those obtained in single cell experiments. These combined studies were not possible prior to the development of KO models. Finally, these models represent critical tools to improve our understanding of the molecular basis of some inheritable arrhythmic human pathologies.
Keywords: HCN KO mouse models; cardiac pacemaking; sinoatrial node.
Figures
Similar articles
-
Deep bradycardia and heart block caused by inducible cardiac-specific knockout of the pacemaker channel gene Hcn4.Proc Natl Acad Sci U S A. 2011 Jan 25;108(4):1705-10. doi: 10.1073/pnas.1010122108. Epub 2011 Jan 10. Proc Natl Acad Sci U S A. 2011. PMID: 21220308 Free PMC article.
-
Novel insights into the distribution of cardiac HCN channels: an expression study in the mouse heart.J Mol Cell Cardiol. 2011 Dec;51(6):997-1006. doi: 10.1016/j.yjmcc.2011.09.005. Epub 2011 Sep 14. J Mol Cell Cardiol. 2011. PMID: 21945247
-
Transcription profiling of HCN-channel isotypes throughout mouse cardiac development.Basic Res Cardiol. 2009 Nov;104(6):621-9. doi: 10.1007/s00395-009-0031-5. Epub 2009 May 7. Basic Res Cardiol. 2009. PMID: 19421833 Free PMC article.
-
HCN-related channelopathies.Pflugers Arch. 2010 Jul;460(2):405-15. doi: 10.1007/s00424-010-0810-8. Epub 2010 Mar 8. Pflugers Arch. 2010. PMID: 20213494 Review.
-
Pacemaker activity of the human sinoatrial node: effects of HCN4 mutations on the hyperpolarization-activated current.Europace. 2014 Mar;16(3):384-95. doi: 10.1093/europace/eut348. Europace. 2014. PMID: 24569893 Review.
Cited by
-
GFP-specific CD8 T cells enable targeted cell depletion and visualization of T-cell interactions.Nat Biotechnol. 2015 Dec;33(12):1287-1292. doi: 10.1038/nbt.3386. Epub 2015 Nov 2. Nat Biotechnol. 2015. PMID: 26524661 Free PMC article.
-
Isoform-specific regulation of HCN4 channels by a family of endoplasmic reticulum proteins.Proc Natl Acad Sci U S A. 2020 Jul 28;117(30):18079-18090. doi: 10.1073/pnas.2006238117. Epub 2020 Jul 9. Proc Natl Acad Sci U S A. 2020. PMID: 32647060 Free PMC article.
-
Speeding Up the Heart? Traditional and New Perspectives on HCN4 Function.Front Physiol. 2021 May 27;12:669029. doi: 10.3389/fphys.2021.669029. eCollection 2021. Front Physiol. 2021. PMID: 34122140 Free PMC article. Review.
-
A detailed characterization of the hyperpolarization-activated "funny" current (If) in human-induced pluripotent stem cell (iPSC)-derived cardiomyocytes with pacemaker activity.Pflugers Arch. 2021 Jul;473(7):1009-1021. doi: 10.1007/s00424-021-02571-w. Epub 2021 May 2. Pflugers Arch. 2021. PMID: 33934225 Free PMC article.
-
Cardiac Pacemaker Activity and Aging.Annu Rev Physiol. 2020 Feb 10;82:21-43. doi: 10.1146/annurev-physiol-021119-034453. Epub 2019 Nov 22. Annu Rev Physiol. 2020. PMID: 31756134 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

