Genetic alteration of stemness factors and p53 in mouse forestomach by chemical carcinogen-induced carcinogenesis
- PMID: 22783421
- PMCID: PMC3392582
- DOI: 10.3892/ol.2012.642
Genetic alteration of stemness factors and p53 in mouse forestomach by chemical carcinogen-induced carcinogenesis
Abstract
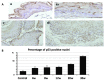
The expression profiles of genes normally enriched in embryonic stem (ES) cells (stemness factors) are associated with poor clinical outcome in solid tumors. However, whether such gene expression is responsible for tumor initiation and progression remains to be determined. The tumor suppressor gene p53 is known to attenuate the expression of Nanog, which is essential for maintaining stem cells in response to DNA damage. On the basis of these findings, we hypothesized that stemness factors and p53 closely correlate with each other and form a network in response to genomic damage in the early phase of carcinogenesis and in the process of tumor progression. In this study, we applied the N-methylbenzylnitrosoamine (NMBA)-induced carcinogenesis model to the mouse forestomach to clarify the role of the stemness factors, c-Myc, Klf4, Sox2, Oct3/4 and Nanog, in cancer development using p53(+/+) (n=26) and p53(+/-) (n=11) C57BL/6J mice. Thirty weeks following NMBA administration, histologically evident squamous cell carcinoma was detected in the forestomachs of p53(+/+) mice, and the percentage of p53-positive nuclei in the forestomach epithelium gradually increased during carcinogenesis. Tumor development in p53(+/-) mice occurred significantly earlier than in p53(+/+) mice. Quantitative real-time PCR analyses revealed a reduced c-Myc and Klf4 expression before evident morphological changes were observed, and an increased expression with the development of squamous cell carcinoma. Sox2 expression remained unchanged until tumor development and increased with the appearance of squamous cell carcinomas. The expression of Oct3/4 and Nanog increased at the early stages following NMBA administration, and Nanog expression in situ was not positively affected by the deficiency of p53. Findings of the present study suggested that Oct3/4 may be involved in the progression of carcinogenesis from normal epithelial cells at early stages, suggesting the potential use of Oct3/4 as a biomarker in forestomach tumor formation at early stages of chemical carcinogenesis.
Figures


Similar articles
-
p53 deficiency accelerates induction and progression of esophageal and forestomach tumors in zinc-deficient mice.Cancer Res. 2003 Jan 1;63(1):186-95. Cancer Res. 2003. PMID: 12517797
-
NTP Toxicology and Carcinogenesis Studies of 1-Amino-2,4-Dibromoanthraquinone (CAS No. 81-49-2) in F344/N Rats and B6C3F1 Mice (Feed Studies).Natl Toxicol Program Tech Rep Ser. 1996 Aug;383:1-370. Natl Toxicol Program Tech Rep Ser. 1996. PMID: 12692653
-
Antizyme overexpression in transgenic mice reduces cell proliferation, increases apoptosis, and reduces N-nitrosomethylbenzylamine-induced forestomach carcinogenesis.Cancer Res. 2003 Jul 15;63(14):3945-54. Cancer Res. 2003. PMID: 12873989
-
Positive immunohistochemical staining of p53 and cyclin D in advanced mouse skin tumors, but not in precancerous lesions produced by benzo[a]pyrene.Carcinogenesis. 1995 Jul;16(7):1629-35. doi: 10.1093/carcin/16.7.1629. Carcinogenesis. 1995. PMID: 7542177
-
SOX2 and p53 Expression Control Converges in PI3K/AKT Signaling with Versatile Implications for Stemness and Cancer.Int J Mol Sci. 2020 Jul 11;21(14):4902. doi: 10.3390/ijms21144902. Int J Mol Sci. 2020. PMID: 32664542 Free PMC article. Review.
Cited by
-
The Establishment of Esophageal Precancerous Lesion Model by Using p53 Conditional Knockout Mouse in Esophageal Epithelium.Biomed Res Int. 2020 Jan 25;2020:4534289. doi: 10.1155/2020/4534289. eCollection 2020. Biomed Res Int. 2020. PMID: 32047812 Free PMC article.
References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Yamanaka S. Elite and stochastic models for induced pluripotent stem cell generation. Nature. 2009;460:49–52. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
