Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease
- PMID: 22784116
- PMCID: PMC3568501
- DOI: 10.1056/NEJMoa1201248
Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease
Abstract
Background: Graft-versus-host disease (GVHD) is a major barrier to successful allogeneic hematopoietic stem-cell transplantation (HSCT). The chemokine receptor CCR5 appears to play a role in alloreactivity. We tested whether CCR5 blockade would be safe and limit GVHD in humans.
Methods: We tested the in vitro effect of the CCR5 antagonist maraviroc on lymphocyte function and chemotaxis. We then enrolled 38 high-risk patients in a single-group phase 1 and 2 study of reduced-intensity allogeneic HSCT that combined maraviroc with standard GVHD prophylaxis.
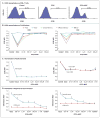
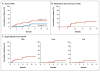
Results: Maraviroc inhibited CCR5 internalization and lymphocyte chemotaxis in vitro without impairing T-cell function or formation of hematopoietic-cell colonies. In 35 patients who could be evaluated, the cumulative incidence rate (±SE) of grade II to IV acute GVHD was low at 14.7±6.2% on day 100 and 23.6±7.4% on day 180. Acute liver and gut GVHD were not observed before day 100 and remained uncommon before day 180, resulting in a low cumulative incidence of grade III or IV GVHD on day 180 (5.9±4.1%). The 1-year rate of death that was not preceded by disease relapse was 11.7±5.6% without excessive rates of relapse or infection. Serum from patients receiving maraviroc prevented CCR5 internalization by CCL5 and blocked T-cell chemotaxis in vitro, providing evidence of antichemotactic activity.
Conclusions: In this study, inhibition of lymphocyte trafficking was a specific and potentially effective new strategy to prevent visceral acute GVHD. (Funded by Pfizer and others; ClinicalTrials.gov number, NCT00948753.).
Conflict of interest statement
Dr. Frey reports receiving lecture fees from Pfizer; Dr. Stadt-mauer, consulting fees from Pfizer; Dr. Vonderheide, grant support from Pfizer; and Dr. Porter, consulting fees from Bristol-Myers Squibb and lecture fees from Celgene and Millennium. No other potential conflict of interest relevant to this article was reported.
Figures

Comment in
-
Blockade of chemotaxis in graft-versus-host disease.N Engl J Med. 2012 Oct 25;367(17):1667; author reply 1667-8. doi: 10.1056/NEJMc1209665. N Engl J Med. 2012. PMID: 23094739 No abstract available.
-
Blockade of CCR5 to protect the liver graft in HIV/HCV co-infected patients.J Hepatol. 2013 Sep;59(3):613-5. doi: 10.1016/j.jhep.2013.03.034. Epub 2013 Apr 9. J Hepatol. 2013. PMID: 23583366 No abstract available.
Similar articles
-
Clinical and immunologic impact of CCR5 blockade in graft-versus-host disease prophylaxis.Blood. 2017 Feb 16;129(7):906-916. doi: 10.1182/blood-2016-08-735076. Epub 2017 Jan 5. Blood. 2017. PMID: 28057639 Free PMC article. Clinical Trial.
-
Extended CCR5 Blockade for Graft-versus-Host Disease Prophylaxis Improves Outcomes of Reduced-Intensity Unrelated Donor Hematopoietic Cell Transplantation: A Phase II Clinical Trial.Biol Blood Marrow Transplant. 2019 Mar;25(3):515-521. doi: 10.1016/j.bbmt.2018.09.034. Epub 2018 Oct 10. Biol Blood Marrow Transplant. 2019. PMID: 30315941 Free PMC article. Clinical Trial.
-
A Pharmacokinetic and Pharmacodynamic Study of Maraviroc as Acute Graft-versus-Host Disease Prophylaxis in Pediatric Allogeneic Stem Cell Transplant Recipients with Nonmalignant Diagnoses.Biol Blood Marrow Transplant. 2016 Oct;22(10):1829-1835. doi: 10.1016/j.bbmt.2016.08.001. Epub 2016 Aug 3. Biol Blood Marrow Transplant. 2016. PMID: 27498124
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
-
C-C chemokine receptor 5 and acute graft-versus-host disease.Immun Inflamm Dis. 2022 Sep;10(9):e687. doi: 10.1002/iid3.687. Immun Inflamm Dis. 2022. PMID: 36039647 Free PMC article. Review.
Cited by
-
MicroRNA-155 Modulates Acute Graft-versus-Host Disease by Impacting T Cell Expansion, Migration, and Effector Function.J Immunol. 2018 Jun 15;200(12):4170-4179. doi: 10.4049/jimmunol.1701465. Epub 2018 May 2. J Immunol. 2018. PMID: 29720426 Free PMC article.
-
Preconditioning serum levels of endothelial cell-derived molecules and the risk of posttransplant complications in patients treated with allogeneic stem cell transplantation.J Transplant. 2014;2014:404096. doi: 10.1155/2014/404096. Epub 2014 Oct 8. J Transplant. 2014. PMID: 25374676 Free PMC article.
-
Low-dose antithymocyte globulin enhanced the efficacy of tacrolimus and mycophenolate for GVHD prophylaxis in recipients of unrelated SCT.Bone Marrow Transplant. 2015 Jan;50(1):106-12. doi: 10.1038/bmt.2014.203. Epub 2014 Oct 6. Bone Marrow Transplant. 2015. PMID: 25285804 Free PMC article.
-
Association between a Suppressive Combined Antiretroviral Therapy Containing Maraviroc and the Hepatitis B Virus Vaccine Response.Antimicrob Agents Chemother. 2017 Dec 21;62(1):e02050-17. doi: 10.1128/AAC.02050-17. Print 2018 Jan. Antimicrob Agents Chemother. 2017. PMID: 29084751 Free PMC article.
-
TNF-receptor inhibitor therapy for the treatment of children with idiopathic pneumonia syndrome. A joint Pediatric Blood and Marrow Transplant Consortium and Children's Oncology Group Study (ASCT0521).Biol Blood Marrow Transplant. 2015 Jan;21(1):67-73. doi: 10.1016/j.bbmt.2014.09.019. Epub 2014 Sep 28. Biol Blood Marrow Transplant. 2015. PMID: 25270958 Free PMC article. Clinical Trial.
References
-
- Johnston L. Acute graft-versus-host disease: differing risk with differing graft sources and conditioning intensity. Best Pract Res Clin Haematol. 2008;21:177–92. - PubMed
-
- Ferrara JL, Levy R, Chao NJ. Pathophysiologic mechanisms of acute graft-vs.-host disease. Biol Blood Marrow Transplant. 1999;5:347–56. - PubMed
-
- Weisdorf D. GVHD the nuts and bolts. Hematology Am Soc Hematol Educ Program. 2007:62–7. - PubMed
-
- Sackstein R. A revision of Billingham's tenets: the central role of lymphocyte migration in acute graft-versus-host disease. Biol Blood Marrow Transplant. 2006;12:2–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
