Uncoupling of eNOS causes superoxide anion production and impairs NO signaling in the cerebral microvessels of hph-1 mice
- PMID: 22784235
- PMCID: PMC3433644
- DOI: 10.1111/j.1471-4159.2012.07872.x
Uncoupling of eNOS causes superoxide anion production and impairs NO signaling in the cerebral microvessels of hph-1 mice
Abstract
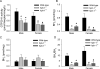
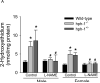
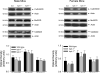
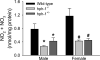
In this study, we used the GTP cyclohydrolase I-deficient mice, i.e., hyperphenylalaninemic (hph-1) mice, to test the hypothesis that the loss of tetrahydrobiopterin (BH(4)) in cerebral microvessels causes endothelial nitric oxide synthase (eNOS) uncoupling, resulting in increased superoxide anion production and inhibition of endothelial nitric oxide signaling. Both homozygous mutant (hph-1(-/-)) and heterozygous mutant (hph-1(+/-) mice) demonstrated reduction in GTP cyclohydrolase I activity and reduced bioavailability of BH(4). In the cerebral microvessels of hph-1(+/-) and hph-1(-/-) mice, increased superoxide anion production was inhibited by supplementation of BH(4) or NOS inhibitor- L- N(G) -nitro arginine-methyl ester, indicative of eNOS uncoupling. Expression of 3-nitrotyrosine was significantly increased, whereas NO production and cGMP levels were significantly reduced. Expressions of antioxidant enzymes namely copper and zinc superoxide dismutase, manganese superoxide dismutase, and catalase were not affected by uncoupling of eNOS. Reduced levels of BH(4), increased superoxide anion production, as well as inhibition of NO signaling were not different between the microvessels of male and female mice. The results of our study are the first to demonstrate that, regardless of gender, reduced BH(4) bioavailability causes eNOS uncoupling, increases superoxide anion production, inhibits eNOS/cGMP signaling, and imposes significant oxidative stress in the cerebral microvasculature.
© 2012 The Authors. Journal of Neurochemistry © 2012 International Society for Neurochemistry.
Figures

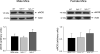



Similar articles
-
Differential effects of eNOS uncoupling on conduit and small arteries in GTP-cyclohydrolase I-deficient hph-1 mice.Am J Physiol Heart Circ Physiol. 2011 Dec;301(6):H2227-34. doi: 10.1152/ajpheart.00588.2011. Epub 2011 Sep 30. Am J Physiol Heart Circ Physiol. 2011. PMID: 21963838 Free PMC article.
-
PPARδ agonist GW501516 prevents uncoupling of endothelial nitric oxide synthase in cerebral microvessels of hph-1 mice.Brain Res. 2012 Nov 5;1483:89-95. doi: 10.1016/j.brainres.2012.09.012. Epub 2012 Sep 13. Brain Res. 2012. PMID: 22982594 Free PMC article.
-
Uncoupling of endothelial nitric oxide synthase in cerebral vasculature of Tg2576 mice.J Neurochem. 2015 Sep;134(6):1129-38. doi: 10.1111/jnc.13205. Epub 2015 Jul 15. J Neurochem. 2015. PMID: 26111938 Free PMC article.
-
Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets?Pharmacol Ther. 2013 Dec;140(3):239-57. doi: 10.1016/j.pharmthera.2013.07.004. Epub 2013 Jul 13. Pharmacol Ther. 2013. PMID: 23859953 Review.
-
Mechanisms and consequences of endothelial nitric oxide synthase dysfunction in hypertension.J Hypertens. 2015 Jun;33(6):1128-36. doi: 10.1097/HJH.0000000000000587. J Hypertens. 2015. PMID: 25882860 Free PMC article. Review.
Cited by
-
Utility of NO and H2S donating platforms in managing COVID-19: Rationale and promise.Nitric Oxide. 2022 Nov 1;128:72-102. doi: 10.1016/j.niox.2022.08.003. Epub 2022 Aug 24. Nitric Oxide. 2022. PMID: 36029975 Free PMC article. Review.
-
Mulberry Extract Attenuates Endothelial Dysfunction through the Regulation of Uncoupling Endothelial Nitric Oxide Synthase in High Fat Diet Rats.Nutrients. 2019 Apr 28;11(5):978. doi: 10.3390/nu11050978. Nutrients. 2019. PMID: 31035424 Free PMC article.
-
Age-Related Modulations of AQP4 and Caveolin-1 in the Hippocampus Predispose the Toxic Effect of Phoneutria nigriventer Spider Venom.Int J Mol Sci. 2016 Nov 23;17(11):1462. doi: 10.3390/ijms17111462. Int J Mol Sci. 2016. PMID: 27886057 Free PMC article.
-
Functional and Structural Improvement with a Catalytic Carbon Nano-Antioxidant in Experimental Traumatic Brain Injury Complicated by Hypotension and Resuscitation.J Neurotrauma. 2019 Jul 1;36(13):2139-2146. doi: 10.1089/neu.2018.6027. Epub 2019 Mar 13. J Neurotrauma. 2019. PMID: 30704349 Free PMC article.
-
Endothelial retinoblastoma protein reduces abdominal aortic aneurysm development via promoting DHFR/NO pathway-mediated vasoprotection.Mol Cell Biochem. 2019 Oct;460(1-2):29-36. doi: 10.1007/s11010-019-03567-y. Epub 2019 Jun 18. Mol Cell Biochem. 2019. PMID: 31214845
References
-
- Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler. Thromb. Vasc. Biol. 2004;24:413–420. - PubMed
-
- Andresen JJ, Faraci FM, Heistad DD. Vasomotor responses in MnSOD-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H1141–1148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

