Polymorphisms in cytochrome P450 2C19 enzyme and cessation of leflunomide in patients with rheumatoid arthritis
- PMID: 22784880
- PMCID: PMC3580556
- DOI: 10.1186/ar3911
Polymorphisms in cytochrome P450 2C19 enzyme and cessation of leflunomide in patients with rheumatoid arthritis
Abstract
Introduction: Rational selection of disease modifying anti-rheumatic drugs in the treatment of rheumatoid arthritis (RA) has many potential advantages, including rapid disease control, reduced long-term disability and reduced overall cost to the healthcare system. Inter-individual genetic differences are particularly attractive as markers to predict efficacy and toxicity, as they can be determined rapidly prior to drug selection. The aims of this study, therefore, were to investigate the association between differences in genes associated with the metabolism, clearance and efficacy of leflunomide with its cessation in a group of rheumatoid arthritis patients who were treated with an intensive contemporary, treat-to-target approach.
Methods: This retrospective cohort study identified all individuals who received leflunomide and were enrolled in the Early Arthritis inception cohort at the Royal Adelaide Hospital between 2001 and July 2011. Inclusion criteria were age (>18) and a diagnosis of rheumatoid arthritis. Patients were excluded if a DNA sample was not available, if they withdrew from the cohort or if clinical data were insufficient. Subjects were followed for 12 months or until either another disease modifying antirheumatic drug was added or leflunomide was ceased. The following single nucleotide polymorphisms (SNPs) were determined: CYP2C19*2 (rs4244285), CYP2C19*17 (rs12248560), ABCG2 421C>A (rs2231142), CYP1A2*1F (rs762551) and DHODH 19C>A (rs3213422). The effects of variables on cessation were assessed with Cox Proportional Hazard models.
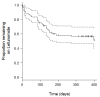
Results: Thirty-three of 78 (42.3%) patients ceased leflunomide due to side effects. A linear trend between cytochrome P450 2C19 (CYP2C19) phenotype and leflunomide cessation was observed, with poor and intermediate metabolizers ceasing more frequently (adjusted Hazard Ratio = 0.432 for each incremental change in phenotype, 95% CI 0.237 to 0.790, P = 0.006). Previously observed associations between cytochrome P450 1A2 (CYP1A2) and dihydro-orotate dehydrogenase (DHODH) genotype and toxicity were not apparent, but there was a trend for ATP-binding cassette sub-family G member 2 (ABCG2) genotype to be associated with cessation due to diarrhea.
Conclusions: CYP2C19 phenotype was associated with cessation due to toxicity, and since CYP2C19 intermediate and poor metabolizers have lower teriflunomide concentrations, it is likely that they have a particularly poor risk:benefit ratio when using this drug.
Figures

References
-
- Proudman SM, Keen HI, Stamp LK, Lee AT, Goldblatt F, Ayres OC, Rischmueller M, James MJ, Hill CL, Caughey GE, Cleland LG. Response-driven combination therapy with conventional disease-modifying antirheumatic drugs can achieve high response rates in early rheumatoid arthritis with minimal glucocorticoid and nonsteroidal anti-inflammatory drug use. Semin Arthritis Rheum. 2007;37:99–111. doi: 10.1016/j.semarthrit.2007.02.001. - DOI - PubMed
-
- Kalgutkar AS, Nguyen HT, Vaz AD, Doan A, Dalvie DK, McLeod DG, Murray JC. In vitro metabolism studies on the isoxazole ring scission in the anti-inflammatory agent lefluonomide to its active alpha-cyanoenol metabolite A771726: mechanistic similarities with the cytochrome P450-catalyzed dehydration of aldoximes. Drug Metab Dispos. 2003;31:1240–1250. doi: 10.1124/dmd.31.10.1240. - DOI - PubMed
-
- Bohanec Grabar P, Grabnar I, Rozman B, Logar D, Tomsic M, Suput D, Trdan T, Peterlin Masic L, Mrhar A, Dolzan V. Investigation of the influence of CYP1A2 and CYP2C19 genetic polymorphism on A771726 pharmacokinetics in leflunomide treated patients with rheumatoid arthritis. Drug Metab Dispos. 2009;37:2061–2068. doi: 10.1124/dmd.109.027482. - DOI - PubMed
-
- Imai Y, Nakane M, Kage K, Tsukahara S, Ishikawa E, Tsuruo T, Miki Y, Sugimoto Y. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Ther. 2002;1:611–616. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

