Results of thoracic endovascular aortic repair 6 years after United States Food and Drug Administration approval
- PMID: 22785216
- PMCID: PMC4089907
- DOI: 10.1016/j.athoracsur.2012.05.072
Results of thoracic endovascular aortic repair 6 years after United States Food and Drug Administration approval
Abstract
Background: Since United States Food and Drug Administration approval in 2005, the short-term safety and efficacy of thoracic endovascular aortic repair (TEVAR) have been established. However, longer-term follow-up data remain lacking. The objective of this study is to report 6-year outcomes of TEVAR in clinical practice.
Methods: A prospective cohort review was performed of all patients undergoing TEVAR at a single referral institution between March 2005 and May 2011. Rates of reintervention were noted. Overall and aortic-specific survival were determined using Kaplan-Meier methods. Log-rank tests were used to compare survival between groups.
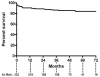
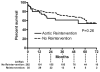
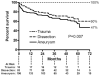
Results: During the study interval, 332 TEVAR procedures were performed in 297 patients. Reintervention was required after 12% of procedures at a mean of 8 ± 14 months after initial TEVAR and was higher in the initial tercile of patients (15.0% vs 9.9%). The 6-year freedom from reintervention was 84%. Type I endoleak was the most common cause of reintervention (5%). Six-year overall survival was 54%, and aorta-specific survival was 92%. Long-term survival was significantly lower than that of an age- and sex-matched United States population (p < 0.001). Survival was similar between patients requiring a reintervention vs those not (p = 0.26). Survival was different based on indication for TEVAR (p = 0.007), and patients with degenerative aneurysms had the lowest survival (47% at 6 years). Cardiopulmonary pathologies were the most common cause of death (27 of 93 total deaths).
Conclusions: Long-term aortic-related survival after TEVAR is high, and the need for reintervention is infrequent. However, overall long-term survival is low, particularly for patients with degenerative aneurysms, and additional work is needed to identify patients unlikely to derive a survival benefit from TEVAR.
Copyright © 2012 The Society of Thoracic Surgeons. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Hughes GC, Daneshmand MA, Balsara KR, et al. “Hybrid” repair of aneurysms of the transverse aortic arch: midterm results. Ann Thorac Surg. 2009;88:1882–7. discussion 1887–8. - PubMed
-
- Hughes GC, Nienaber JJ, Bush EL, Daneshmand MA, McCann RL. Use of custom Dacron branch grafts for “hybrid” aortic debranching during endovascular repair of thoracic and thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2008;136:21–8. 28.e1–6. - PubMed
-
- Lee WA, Daniels MJ, Beaver TM, Klodell CT, Raghinaru DE, Hess PJ., Jr Late outcomes of a single-center experience of 400 consecutive thoracic endovascular aortic repairs. Circulation. 2011;123:2938–45. - PubMed
-
- Geisbusch P, Hoffmann S, Kotelis D, Able T, Hyhlik-Dürr A, Böckler D. Reinterventions during midterm follow-up after endovascular treatment of thoracic aortic disease. J Vasc Surg. 2011;53:1528–33. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

