Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens
- PMID: 22785313
- PMCID: PMC3397405
- DOI: 10.1038/nature11160
Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens
Abstract
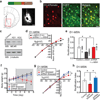
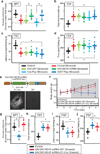
Chronic stress is a strong diathesis for depression in humans and is used to generate animal models of depression. It commonly leads to several major symptoms of depression, including dysregulated feeding behaviour, anhedonia and behavioural despair. Although hypotheses defining the neural pathophysiology of depression have been proposed, the critical synaptic adaptations in key brain circuits that mediate stress-induced depressive symptoms remain poorly understood. Here we show that chronic stress in mice decreases the strength of excitatory synapses on D1 dopamine receptor-expressing nucleus accumbens medium spiny neurons owing to activation of the melanocortin 4 receptor. Stress-elicited increases in behavioural measurements of anhedonia, but not increases in measurements of behavioural despair, are prevented by blocking these melanocortin 4 receptor-mediated synaptic changes in vivo. These results establish that stress-elicited anhedonia requires a neuropeptide-triggered, cell-type-specific synaptic adaptation in the nucleus accumbens and that distinct circuit adaptations mediate other major symptoms of stress-elicited depression.
Figures




Comment in
-
Losing the lust for life: a new role for an old feeding Peptide?Neuron. 2012 Aug 9;75(3):360-2. doi: 10.1016/j.neuron.2012.07.018. Neuron. 2012. PMID: 22884320
References
-
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 2005;8:571–578. - PubMed
-
- Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu. Rev. Neurosci. 2007;30:367–398. - PubMed
-
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

