Inhaled corticosteroids for stable chronic obstructive pulmonary disease
- PMID: 22786484
- PMCID: PMC8992433
- DOI: 10.1002/14651858.CD002991.pub3
Inhaled corticosteroids for stable chronic obstructive pulmonary disease
Update in
-
Inhaled corticosteroids versus placebo for stable chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2023 Mar 27;3(3):CD002991. doi: 10.1002/14651858.CD002991.pub4. Cochrane Database Syst Rev. 2023. PMID: 36971693 Free PMC article.
Abstract
Background: The role of inhaled corticosteroids (ICS) in chronic obstructive pulmonary disease (COPD) has been the subject of much controversy. Major international guidelines recommend selective use of ICS. Recently published meta-analyses have reported conflicting findings on the effects of inhaled steroid therapy in COPD.
Objectives: To determine the efficacy and safety of inhaled corticosteroids in stable patients with COPD, in terms of objective and subjective outcomes.
Search methods: A pre-defined search strategy was used to search the Cochrane Airways Group Specialised Register for relevant literature. Searches are current as of July 2011.
Selection criteria: We included randomised trials comparing any dose of any type of inhaled steroid with a placebo control in patients with COPD. Acute bronchodilator reversibility to short-term beta(2)-agonists and bronchial hyper-responsiveness were not exclusion criteria. The a priori primary outcome was change in lung function. We also analysed data on mortality, exacerbations, quality of life and symptoms, rescue bronchodilator use, exercise capacity, biomarkers and safety.
Data collection and analysis: Two review authors independently assessed trial quality and extracted data. We contacted study authors for additional information. We collected adverse effects information from the trials.
Main results: Fifty-five primary studies with 16,154 participants met the inclusion criteria. Long-term use of ICS (more than six months) did not consistently reduce the rate of decline in forced expiratory volume in one second (FEV(1)) in COPD patients (generic inverse variance analysis: mean difference (MD) 5.80 mL/year with ICS over placebo, 95% confidence interval (CI) -0.28 to 11.88, 2333 participants; pooled means analysis: 6.88 mL/year, 95% CI 1.80 to 11.96, 4823 participants), although one major trial demonstrated a statistically significant difference. There was no statistically significant effect on mortality in COPD patients (odds ratio (OR) 0.98, 95% CI 0.83 to 1.16, 8390 participants). Long-term use of ICS reduced the mean rate of exacerbations in those studies where pooling of data was possible (generic inverse variance analysis: MD -0.26 exacerbations per patient per year, 95% CI -0.37 to -0.14, 2586 participants; pooled means analysis: MD -0.19 exacerbations per patient per year, 95% CI -0.30 to -0.08, 2253 participants). ICS slowed the rate of decline in quality of life, as measured by the St George's Respiratory Questionnaire (MD -1.22 units/year, 95% CI -1.83 to -0.60, 2507 participants). Response to ICS was not predicted by oral steroid response, bronchodilator reversibility or bronchial hyper-responsiveness in COPD patients. There was an increased risk of oropharyngeal candidiasis (OR 2.65, 95% CI 2.03 to 3.46, 5586 participants) and hoarseness. In the long-term studies, the rate of pneumonia was increased in the ICS group compared to placebo, in studies that reported pneumonia as an adverse event (OR 1.56, 95% CI 1.30 to 1.86, 6235 participants). The long-term studies that measured bone effects generally showed no major effect on fractures and bone mineral density over three years.
Authors' conclusions: Patients and clinicians should balance the potential benefits of inhaled steroids in COPD (reduced rate of exacerbations, reduced rate of decline in quality of life and possibly reduced rate of decline in FEV(1)) against the potential side effects (oropharyngeal candidiasis and hoarseness, and risk of pneumonia).
Conflict of interest statement
Ian Yang, Kwun Fong, Melissa Clarke, Esther Sim: none declared.
Figures










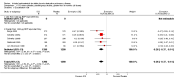

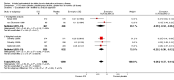






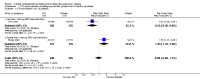

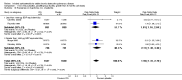




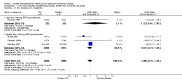











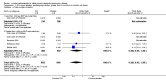









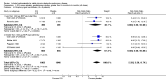

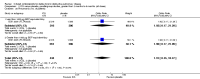




























Update of
-
Inhaled corticosteroids for stable chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2007 Apr 18;(2):CD002991. doi: 10.1002/14651858.CD002991.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2012 Jul 11;(7):CD002991. doi: 10.1002/14651858.CD002991.pub3. PMID: 17443520 Updated.
References
References to studies included in this review
Auffarth 1991 {published data only}
Boothman‐Burrell 1997 {published data only}
-
- Boothman‐Burrell D, Delany SG, Flannery EM, Hancox RJ, Taylor DR. The efficacy of inhaled corticosteroids in the management of non asthmatic chronic airflow obstruction. New Zealand Medical Journal 1997;110(1053):370‐3. - PubMed
Bourbeau 1998 {published data only}
Bourbeau 2007 {published data only}
Brightling 2005 {published data only}
Burge 2000 {published data only}
-
- Bale G, Martinez‐Camblor P, Burge PS, Soriano JB. Long‐term mortality follow‐up of the ISOLDE participants: causes of death during 13 years after trial completion. Respiratory Medicine 2008;102(10):1468‐72. - PubMed
-
- Calverley PM, Spencer S, Willits L, Burge PS, Jones PW. Withdrawal from treatment as an outcome in the ISOLDE study of COPD. Chest 2003;124(4):1350. - PubMed
Calverley 2003a {published data only}
-
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003;361(9356):449‐56. - PubMed
-
- Calverley PMA, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Safety of salmeterol/fluticasone propionate combination in the treatment of chronic obstructive pulmonary disease. European Respiratory Journal 2002;20(Suppl 38):242s [P1572].
-
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Clinical improvements with salmeterol / fluticasone propionate combination in differing severities of COPD [A035] [Poster D50]. http://www.abstracts2view.com 2003.
-
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Salmeterol/fluticasone propionate combination for one year provides greater clinical benefit than its individual components [A98] [Poster 306]. http://www.abstracts‐on‐line.com/abstracts/ATS 2002.
-
- Hunjan MK, Chandler F. Numbers needed to treat (NNT) to avoid an exacerbation in patients with chronic obstructive pulmonary disease (COPD) using salmeterol/fluticasone propionate combination (SFC) and associated costs [Abstract]. American Thoracic Society 100th International Conference, May 21‐26. 2004:D22 Poster 503.
Calverley 2003b {published data only}
-
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]. American Thoracic Society 100th International Conference, May 21‐26 2004;(no volume):C22 Poster 505.
-
- Calverley PM, Bonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. European Respiratory Journal 2003;22(6):912‐9. - PubMed
-
- Calverley PMA, Cseke Z, Peterson S. Budesonide/formoterol reduces the use of oral corticosteroids in the treatment of COPD [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P436. - PubMed
-
- Calverley PMA, Kuna P, Olsson H. COPD exacerbations are reduced by budesonide/formoterol in a single inhaler [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P1587.
-
- Calverley PMA, Olsson H, Symbicort International COPD Study Group. Budesonide/formoterol in a single inhaler sustains improvements in lung function over 12 months compared with monocomponents and placebo in patients with COPD [abstract]. American Thoracic Society 99th International Conference. 2003:B024 Poster 418.
Calverley 2003c {unpublished data only}
-
- Calverley P, Pauwels R, Nieminem M, Stryszak P, Staudinger H, Lee T. Once‐daily mometasone furoate dry powder inhaler preserves lung function, reduces symptoms, and delays exacerbations in patients with COPD previously maintained on ICS. 13th ERS Annual Congress, 27 September, 2003, Vienna. 2003:P155.
Calverley 2007 {unpublished data only}
-
- Calverley P, Celli B, Anderson JA, Ferguson GT, Jenkins C, Jones PW, et al. The TOwards a Revolution in COPD Health (TORCH) study: salmeterol/fluticasone propionate improves survival in COPD over three years [Abstract]. Respirology 2006;11(Suppl 5):A149 [PS‐3‐8].
-
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW et al and TORCH = Investigators. Cardiovascular events in patients with COPD: TORCH study results. Thorax 2010;65(8):719‐25. - PubMed
-
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. New England Journal of Medicine 2007;356(8):775‐89. - PubMed
-
- Calverley PM, Celli B, Anderson JA, Ferguson GT, Jenkins C, Jones PW, Vestbo J, et al. The towards a revolution in COPD health (TORCH) study: fluticasone propionate/salmeterol improves survival in COPD over three years [Abstract]. Chest 2006;130(4):122s.
-
- Calverley PMA, Celli B, Andersen JA, Ferguson GT, Jenkins C, Jones PW, et al. The TORCH (towards a revolution in COPD health) study salmeterol/fluticasone propionate (SFC) improves survival in COPD over three years [Abstract]. European Respiratory Journal 2006;28(Suppl 50):34s [E311].
Calverley 2008 {published data only}
-
- Nelson H, Karpel JP, Staudinger H, Busse WW. Mometasone furoate dry powder inhaler (MF‐DPI) improves FEV1, symptoms, quality of life and reduces exacerbations in patients with COPD. Chest 2004;236(4 Suppl):709s‐10s.
Culpitt 1999 {published data only}
-
- Culpitt SV, Maziak W, Loukidis S, Nightingale JA, Matthews JL, Barnes PJ. Effect of high dose inhaled steroid on cells, cytokines, and proteases in induced sputum in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1999;160(5 Pt 1):1635‐9. - PubMed
Derenne 1995 {published data only}
-
- Derenne JP. Effects of high dose inhaled beclomethasone in the rate of decline in FEV1 in patients with chronic obstructive pulmonary disease: results of a 2 years prospective multicentre study. American Journal of Respiratory and Critical Care Medicine 1995;151:A463.
Ferreira 2001 {published data only}
-
- Ferreira IM, Hazari MS, Gutierrez C, Zamel N, Chapman KR. Exhaled nitric oxide and hydrogen peroxide in patients with chronic obstructive pulmonary disease: effects of inhaled beclomethasone. American Journal of Respiratory and Critical Care Medicine 2001;164(6):1012‐5. - PubMed
Ferreira 2003 {unpublished data only}
-
- Ferreira IM, Sandrini A, Zamel N, Balter M, Chapman KR. Effects of inhaled fluticasone propionate (FP) on Exhaled Nitric Oxide (ENO), functional exercise capacity and quality of life in stable patients with COPD. American Thoracic Society 99th International Conference. 2003:B024 Poster 404.
GSK 2005 (FCO30002) {unpublished data only}
-
- FCO30002. A multicentre, randomised, placebo‐controlled, double‐blind comparison with 3 parallel groups to investigate the efficacy and safety of inhaled glucocorticoid fluticasone (500 μg bd via Diskus™) vs. oral glucocorticoid therapy vs. placebo in subjects with chronic obstructive airway disease (COPD) under therapy with Salmeterol (50 μg bd). GlaxoSmithKline Clinical Trial Register 2005.
GSK 2005 (FLTA3025) {unpublished data only}
-
- FLTA3025. A randomized, double‐blind, parallel‐group, comparative trial of inhaled fluticasone propionate 250mcg BID, 500mcg BID, and placebo BID via the DISKUS in subjects with chronic obstructive pulmonary disease (COPD). GlaxoSmithKline Clinical Trial Register 2005.
Guenette 2011 {published data only}
-
- Guenette JA, Raghavan N, Harris‐McAllister V, Preston ME, Webb KA, O'Donnell DE. Effect of adjunct fluticasone propionate on airway physiology during rest and exercise in COPD. Respiratory Medicine. 2011/09/16 2011; Vol. 105, issue 12:1836‐45. [1532‐3064: (Electronic)] - PubMed
Hanania 2003 {published data only}
-
- Hanania NA, Darken P, Horstman D, Reisner C, Lee B, Davis S, et al. The efficacy and safety of fluticasone propionate (250 micro g)/salmeterol (50 micro g) combined in the Diskus inhaler for the treatment of COPD. Chest 2003;124(3):834‐43. - PubMed
-
- Hanania NA, Ramsdell J, Payne K, Davis S, Horstman D, Lee B, et al. Improvements in airflow and dyspnea in COPD patients following 24 weeks treatment with salmeterol 50mcg and fluticasone propionate 250mcg alone or in combination via the Diskus. American Journal of Respiratory and Critical Care Medicine 2001;163(Suppl 5):A279.
-
- Horstman D, Darken P, Davis S, Lee B. Improvements in FEV1 and symptoms in poorly reversible COPD patients following treatment with salmeterol 50mcg/fluticasone propionate 250mcg combination [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P434.
-
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to combination therapy in chronic obstructive pulmonary disease (COPD) [Abstract]. National COPD Conference; Arlington, Virginia. 2003:1081.
-
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to salmeterol/fluticasone propionate combination therapy in COPD [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P429.
Hattotuwa 2002 {published data only}
-
- Hattotuwa KL, Gizycki MJ, Ansari TW, Jeffery PK, Barnes NC. The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2002;165(12):1592‐9. - PubMed
John 2005 {published data only}
-
- John M, Bosse S, Oltmanns U, Schumacher A, Witt C. Effects of inhaled HFA beclomethasone on pulmonary function and symptoms in patients with chronic obstructive pulmonary disease. Respiratory Medicine 2005;99(11):1418‐24. - PubMed
-
- John M, Bosse S, Schumacher A, Oltmanns U, Witt C. Effects of inhaled beclomethasone HFA 134 (qvar/ventoiair) on health related quality of life in patients with chronic obstructive pulmonary disease [Abstract]. American Thoracic Society 2005 International Conference. 2005:Poster A43.
Kerstjens 1992 {published data only}
-
- Kaptein AA, Brand PL, Dekker FW, Kerstjens HA, Postma DS, Sluiter HJ. Quality‐of‐life in a long‐term multicentre trial in chronic nonspecific lung disease: assessment at baseline. The Dutch CNSLD Study Group. European Respiratory Journal 1993;6(10):1479‐84. - PubMed
-
- Kerstjens HA, Overbeek SE, Schouten JP, Brand PL, Postma DS. Airways hyperresponsivenes, bronchodilator response, allergy and smoking predict improvement in FEV1 during long‐term inhaled corticosteroid treatment. Dutch CNSLD Study Group. European Respiratory Journal 1993;6(6):868‐76. - PubMed
Lapperre 2009 {published data only}
-
- Lapperre TS, Snoeck‐Stroband JB, Gosman MM, Jansen DF, Schadewijk A, Thiadens HA, et al. Groningen Leiden Universities Corticosteroids in Obstructive Lung Disease Study Group. Effect of fluticasone with and without salmeterol on pulmonary outcomes in chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine 2009;151(8):517‐27. - PubMed
Laptseva 2002 {published data only}
-
- Laptseva IM, Laptseva EA, Borshchevsky VV, Gurevich G, Kalechits O. Inhaled budesonide in the management of chronic obstructive pulmonary disease. European Respiratory Journal 2002;20(Suppl 38):244s.
LHS 2000 {published data only}
-
- Anthonisen NR, Connett JC, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. American Journal of Respiratory and Critical Care Medicine 2002;166(5):675‐9. - PubMed
-
- Eichenhorn MS, Wise RA, Madhok TC, Gerald LB, Bailey WC, Tashkin DP, et al. Lack of long‐term adverse adrenal effects from inhaled triamcinolone: Lung Health Study II. Chest 2003;124(1):57‐62. - PubMed
-
- Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. New England Journal of Medicine 2000;343(26):1902‐9. - PubMed
-
- Scanlon PD, Connett JE, Wise RA, Tashkin DP, Madhok T, Skeans M, et al. Loss of one density with inhaled triamcinolone in Lung Health Study II. American Journal of Respiratory and Critical Care Medicine 2004;170(12):1302‐9. - PubMed
-
- Tashkin DP, Murray HE, Skeans M, Murray RP. Skin manifestations of inhaled corticosteroids in COPD patients. Chest 2004;126(4):1123‐33. - PubMed
Llewellyn‐Jones 1996 {published data only}
-
- Llewellyn‐Jones CG, Harris TA, Stockley RA. Effect of fluticasone propionate on sputum of patients with chronic bronchitis and emphysema. American Journal of Respiratory and Critical Care Medicine 1996;153(2):616‐21. - PubMed
Loppow 2001 {published data only}
-
- Loppow D, Schleiss MB, Kanniess F, Taube C, Jorres RA, Magnussen H. In patients with chronic bronchitis a four week trial with inhaled steroids does not attenuate airway inflammation. Respiratory Medicine 2001;95(2):115‐21. - PubMed
Mahler 2002 {published data only}
-
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to combination therapy in chronic obstructive pulmonary disease (COPD). http://www.abstracts2view.com 2003.
-
- Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2002;166(8):1084‐91. - PubMed
-
- SFCA3006. A randomized, double‐blind, placebo‐controlled, parallel‐group trial evaluating the safety and efficacy of the DISKUS formulations of salmeterol (SAL) 50mcg BID and fluticasone propionate (FP) 500mcg BID individually and in combination as salmeterol 50mcg/fluticasone propionate 500mcg BID (SFC 50/500) compared to placebo in COPD subjects. http://ctr.gsk.co.uk 2004.
Mirici 2001 {published data only}
-
- Mirici A, Bektas Y, Ozbakis G, Erman Z. Effect of inhaled corticosteroids on respiratory function tests and airway inflammation in stable chronic obstructive pulmonary disease. Clinical Drug Investigation 2001;21(12):835‐42.
Nishimura 1999 {published data only}
-
- Nishimura K, Koyama H, Ikeda A, Tsukino M, Hajiro T, Mishima M, et al. The effect of high‐dose inhaled beclomethasone dipropionate in patients with stable COPD. Chest 1999;115(1):31‐7. - PubMed
Ozol 2005 {published data only}
-
- Ozol D, Aysan T, Solak ZA, Mogulkoc N, Veral A, Sebik F. The effect of inhaled corticosteroids on bronchoalveolar lavage cells and IL‐8 levels in stable COPD patients. Respiratory Medicine 2005;99(12):1494‐500. - PubMed
Paggiaro 1998 {published data only}
-
- FLIT97. A multi‐centre, randomised, double‐blind, parallel group study of the efficacy and safety of inhaled fluticasone propionate 1000µg daily with placebo in chronic obstructive pulmonary disease. http://ctr.gsk.co.uk 2005.
-
- Paggiaro PL, Dahle R, Bakran I, Frith L, Hollingworth K, Efthimiou J. Multicentre randomised placebo‐controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. International COPD Study Group. Lancet 1998;351(9105):773‐80. - PubMed
Pauwels 1999 {published data only}
-
- Johnell O, Pauwels R, Löfdahl C‐G, Laitinen LA, Postma DS, Pride NB, et al. Bone mineral density in patients with chronic obstructive pulmonary disease treated with budesonide Turbuhaler. European Respiratory Journal 2002;19(6):1058‐63. - PubMed
-
- Lofdahl CG, Postma DS, Laitinen LA, Ohlsson SV, Pauwels RA, Pride NB. The European Respiratory Society study on chronic obstructive pulmonary disease (EUROSCOP): recruitment methods and strategies. Respiratory Medicine 1998;92(3):467‐72. - PubMed
-
- Pauwels RA, Lofdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, et al. Long‐term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. New England Journal of Medicine 1999;340(25):1948‐53. - PubMed
-
- Pauwels RA, Lofdahl CG, Pride NB, Postma DS, Laitinen LA, Ohlsson SV. European Respiratory Society study on chronic obstructive pulmonary disease (EUROSCOP): hypothesis and design. European Respiratory Journal 1992;5(10):1254‐61. - PubMed
Renkema 1996 {published data only}
-
- Renkema TE, Schouten JP, Koeter GH, Postma DS. Effects of long‐term treatment with corticosteroids in COPD. Chest 1996;109(5):1156‐62. - PubMed
Robertson 1986 {published data only}
-
- Robertson AS, Gove RI, Wieland GA, Burge PS. A double‐blind comparison of oral prednisolone 40 mg/day with inhaled beclomethasone dipropionate 1500 ug/day in patients with adult onset chronic obstructive airways disease. European Journal of Respiratory Diseases 1986;146 Suppl:565‐9. - PubMed
Rutgers 1998 {published data only}
-
- Rutgers SR, Koeter GH, Mark TW, Postma DS. Short‐term treatment with budesonide does not improve hyperresponsiveness to adenosine 5'‐monophosphate in COPD. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Pt 1):880‐6. - PubMed
Schermer 2009 {published data only}
-
- Schermer T, Chavannes N, Dekhuijzen R, Wouters E, Muris J, Akkermans R, et al. Fluticasone and N‐acetylcysteine in primary care patients with COPD or chronic bronchitis. Respiratory Medicine 2009;103(4):542‐51. - PubMed
SCO30002 2005 {unpublished data only}
-
- SCO 30002. A multicentre, randomised, double‐blind, parallel group, placebo‐controlled study to compare the efficacy and safety of inhaled salmeterol/fluticasone propionate combination product 25/250 µg two puffs bd and fluticasone propionate 250µg two puffs bd alone, all administered via metered dose inhalers (MDI), in the treatment of subjects with chronic obstructive pulmonary disease (COPD) for 52 weeks. GlaxoSmithKline Clinical Trial Register 2005.
Senderovitz 1999 {published data only}
-
- Senderovitz T, Vestbo J, Frandsen J, Maltbaek N, Norgaard M, Nielsen C, et al. Steroid reversibility test followed by inhaled budesonide or placebo in outpatients with stable chronic obstructive pulmonary disease. Respiratory Medicine 1999;93(10):715‐8. - PubMed
Shaker 2009 {published data only}
-
- Shaker SB, Dirksen A, Ulrik CS, Hestad M, Stavngaard T, Laursen LC, Maltbaek N, et al. The effect of inhaled corticosteroids on the development of emphysema in smokers assessed by annual computed tomography. COPD: Journal of Chronic Obstructive Pulmonary Disease 2009;6(2):104‐11. - PubMed
-
- Shaker SB, Stavngaard T, Laursen LC, Stoel BC, Dirksen A. Rapid fall in lung density following smoking cessation in COPD. COPD: Journal of Chronic Obstructive Pulmonary Disease 2011;8(1):2‐7. - PubMed
Sin 2004 {published data only}
-
- Sin DD, Lacy P, York E, Man SFP. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2004;170(7):760‐5. - PubMed
Sin 2008 {published data only}
-
- Sin DD, Man SF, Marciniuk DD, Ford G, FitzGerald M, Wong E, York E, et al. ABC (Advair, Biomarkers in COPD) Investigators. The effects of fluticasone with or without salmeterol on systemic biomarkers of inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2008;177(11):1207‐14. - PubMed
Szafranski 2003 {published data only}
-
- Anderson P. Budesonide/formoterol in a single inhaler (Symbicort) provides early and sustained improvement in lung function in moderate to severe COPD. Thorax 2002;57(Suppl III):iii43.
-
- Calverley PMA. Effect of budesonide/formoterol on severe exacerbations and lung function in moderate to severe COPD. Thorax 2002;BTS Winter Meeting 2002:S145.
-
- Calverley PMA, Thompson NC, Olsson H. Budesonide/formoterol in a single inhaler sustains lung function improvements in COPD [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P435.
-
- Campbell LM, Szafranski W. Budesonide/formoterol in a single inhaler (Symbicort) provides sustained relief from symptoms in moderate to severe COPD. Thorax. 2002; Vol. BTS Winter Meeting 2002:S143.
-
- Campbell LW, Szafranski W. Budesonide/formoterol in a single inhaler (Symbicort) reduces severe exacerbations in patients with moderate‐severe COPD. Thorax. 2002; Vol. BTS Winter Meeting 2002:S141.
Tashkin 2008 {published data only}
-
- Tashkin DP, Rennard SI, Martin P, Ramachandran S, Martin UJ, Silkoff PE, et al. SHINE (Division of Pulmonary, Critical Care Medicine. University of California, Los Angeles, California, USA). Efficacy and safety of budesonide and formoterol in one pressurized metered‐dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6‐month randomized clinical trial. Drugs 2008;68(14):1975‐2000. - PubMed
Thompson 1992 {published data only}
-
- Thompson AB, Mueller MB, Heires AJ, Bohling TL, Daughton D, Yancey SW, et al. Aerosolized beclomethasone in chronic bronchitis. Improved pulmonary function and diminished airway inflammation. American Review of Respiratory Disease 1992;146(2):389‐95. [MEDLINE: ] - PubMed
Thompson 2002 {published data only}
-
- Thompson WH, Carvalho P, Souza JP, Charan NB. Controlled trial of inhaled fluticasone propionate in moderate to severe COPD. Lung 2002;180(4):191‐201. - PubMed
van Grunsven 1999 {published data only}
van Grunsven 2003 {published data only}
-
- Grunsven P, Schermer T, Akkermans R, Albers M, Boom G, Schayck O, et al. Short‐ and long‐term efficacy of fluticasone propionate in subjects with early signs and symptoms of chronic obstructive pulmonary disease. Results of the DIMCA study. Respiratory Medicine 2003;97(12):1303‐12. - PubMed
Verhoeven 2002 {published data only}
Vestbo 1999 {published data only}
-
- Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long‐term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 1999;353(9167):1819‐23. [MEDLINE: ] - PubMed
Weiner 1995 {published data only}
-
- Weiner P, Weiner M, Azgad Y, Zamir D. Inhaled budesonide therapy for patients with stable COPD. Chest 1995;108(6):1568‐71. - PubMed
Weiner 1999 {published data only}
-
- Weiner P, Weiner M, Rabner M, Waizman J, Magadle R, Zamir D. The response to inhaled and oral steroids in patients with stable chronic obstructive pulmonary disease. Journal of Internal Medicine 1999;245(1):83‐9. - PubMed
Weir 1990a {published data only}
-
- Weir DC, Gove RI, Robertson AS, Burge PS. Response to corticosteroids in chronic airflow obstruction: relationship to emphysema and airways collapse. European Respiratory Journal 1991;4(10):1185‐90. - PubMed
Weir 1999 {published data only}
-
- Weir DC, Bale GA, Bright P, Sherwood Burge P. A double‐blind placebo‐controlled study of the effect of inhaled beclomethasone dipropionate for 2 years in patients with nonasthmatic chronic obstructive pulmonary disease. Clinical & Experimental Allergy Supplement 1999;29(2):125‐8. - PubMed
Wempe 1992 {published data only}
Yildiz 2004 {published data only}
-
- Yildiz F, Basyigit I, Yildirim E, Boyaci H, Ilgazli A. Does addition of inhaled steroids to combined bronchodilator therapy affect health status in patients with COPD?. Respirology 2004;9(3):352‐5. - PubMed
References to studies excluded from this review
Albers 2004 {published data only}
-
- Albers M, Schermer T, dan Boom G, Akkermans R, Schayck C, Herwaarden C, et al. Efficacy of inhaled steroids in undiagnosed subjects at high risk for COPD: results of the detection, intervention, and monitoring of COPD and asthma program. Chest 2004;126(6):1815‐24. - PubMed
Anonymous 1999 {published data only}
-
- Anonymous. Inhaled glucocorticoids do not help smokers with mild chronic obstructive pulmonary disease. Modern Medicine of Australia 1999;42(12):9.
Anonymous 2000 {published data only}
-
- Anonymous. Inhaled corticosteroids: their role in chronic obstructive pulmonary disease. MeReC Bulletin 2000;11(6):21‐4.
Balbi 2000 {published data only}
-
- Balbi B, Majori M, Bertacco S, Convertino G, Cuomo A, Donner CF, et al. Inhaled corticosteroids in stable COPD patients: do they have effects on cells and molecular mediators of airway inflammation?. Chest 2000;117(6):1633‐7. - PubMed
Bensch 2003 {published data only}
-
- Bensch G, Hampel F, Sachs H. Once‐daily, evening administration of mometasone furoate dry powder inhaler improves pulmonary function in patients with mild to moderate persistent asthma. European Respiratory Journal 2003;22(Suppl 45):282s.
Burge 1999 {published data only}
Chan 1993 {published data only}
-
- Chan CHS, Cohen M, Pang J. The effects of inhaled corticosteroids on chronic airflow limitation. Asian Pacific Journal of Allergy and Immunology 1993;11(2):97‐101. - PubMed
Confalonieri 1998 {published data only}
Corda 2008 {published data only}
-
- Corda L, Bertella E, Piana GE, Boni E, Redolfi S, Tantucci C. Inhaled corticosteroids as additional treatment in alpha‐1‐antitrypsin‐deficiency‐related COPD. Respiration 2008;76(1):61‐8. - PubMed
Cox 1999 {published data only}
-
- Cox G, Whitehead L, Dolovich M, Jordana M, Gauldie J, Newhouse MT. A randomised controlled trial on the effect of inhaled corticosteroids on airways inflammation in adult cigarette smokers. Chest 1999;115(5):1271‐7. - PubMed
Dompeling 1992 {published data only}
-
- Dompeling E, Schayck CP, Molema J, Folgering H, Grunsven PM, Weel C. Inhaled beclomethasone improves the course of asthma and COPD. European Respiratory Journal 1992;5(8):945‐52. - PubMed
Dompeling 1993 {published data only}
-
- Dompeling E, Schayck CP, Grunsven PM, Herwaarden CLA, Akkermans R, Molema J, et al. Slowing the deterioration of asthma and chronic obstructive pulmonary disease observed during bronchodilator therapy by adding inhaled corticosteroids. Annals of Internal Medicine 1993;118(10):770‐8. - PubMed
Egan 1999 {published and unpublished data}
-
- Egan JJ, Maden C, Kalra S, Adams JE, Eastell R, Woodcock AA. A randomised, double blind study comparing the effects of beclomethasone and fluticasone on bone density over 2 years. European Respiratory Journal 1999;13(6):1267‐75. - PubMed
Engel 1989 {published data only}
-
- Engel T, Heinig JH, Madsen O, Hansen M, Weeke ER. A trial of inhaled budesonide on airway responsiveness in smokers with chronic bronchitis. European Respiratory Journal 1989;2(10):935‐9. - PubMed
Fattore 2005 {published data only}
-
- Fattore G, Torbica A, Mangone M. Cost‐analysis of four treatment strategies in the management of moderate‐to‐severe COPD: an application non‐parametric bootstrap. Pharmacoeconomics Italian Research Articles 2005;72(2):135‐43.
Fazio 1986 {published data only}
-
- Fazio F, Lafortuna CL. Beclomethasone dipropionate does not affect mucociliary clearance in patients with chronic obstructive lung disease. Respiration 1986;50(1):62‐5. - PubMed
Guleria 2003 {published data only}
-
- Guleria R, Singh TR, Sinha S, Padhy K, Gupta K, Pande JN. Effect of single inhalation of a salbutamol, ipratropium bromide and beclomethasone on mucociliary clearance in patients with chronic obstructive airway disease. Indian Journal of Chest Diseases & Allied Sciences 2003;45(4):241‐6. - PubMed
Keatings 1997 {published data only}
-
- Keatings VM, Jatakanon A, Worsdell YM, Barnes PJ. Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPD. American Journal of Respiratory and Critical Care Medicine 1997;155:542‐8. - PubMed
Kozak‐Skzopek 1997 {published data only}
-
- Kozak‐Skzopek E, Ulmer WT. Inhalative Budesonid‐Therapie bei chronischer Bronchitis. Atemweg und Lungenerkrankheiten 1997;23(9):542‐6.
Matlin 1976 {unpublished data only}
-
- Matlin RA. The efficacy of steroid aerosol in chronic obstructive pulmonary disease (COPD). American Review of Respiratory Disease 1976;113(4):184.
Melani 1999 {published data only}
-
- Melani AS, Gregorio A. Four‐week nebulized beclomethasone dipropionate in stable COPD patients with exertional dyspnoea. Monaldi Archives for Chest Disease 1999;54(3):224‐7. - PubMed
Moller 1999 {published data only}
-
- Moller M, Haller S, Kiebart M, Cloes R. AeroBec Autohaler in patients with reversible chronic‐obstructive respiratory diseases. Atemwegs‐ Und Lungenkrankheiten 1999;25(3):160‐7.
Nava 2000 {published data only}
-
- Nava S, Compagnoni ML. Controlled short‐term trial of fluticasone propionate in ventilator‐dependent patients with COPD. Chest 2000;118(4):990‐9. - PubMed
Nishimura 2000 {published data only}
-
- Nishimura K, Ikeda A, Koyama H, Zhang M, Tsukino M, Hajiro T, et al. Additive effects of prednisolone and beclomethasone dipropionate in patients with stable chronic obstructive pulmonary disease. Pulmonary Pharmacology & Therapeutics 2000;13(5):225‐30. - PubMed
O'Brien 2001 {published data only}
-
- O'Brien A, Russo‐Magno P, Karki A, Hiranniramol S, Hardin M, Kaszuba M, et al. Effects of withdrawal of inhaled steroids in men with severe irreversible airflow obstruction. American Journal of Respiratory and Critical Care Medicine 2001;164(3):365‐71. - PubMed
Ouyang 1998 {published data only}
-
- Ouyang R, Yin B. Clinical study of inhaled corticosteroid in non‐asthmatic chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi 1998;21(8):497‐9. - PubMed
Roth 1996 {published data only}
-
- Roth R. Inhaled corticosteroids are effective and well tolerated [Inhalative Glukokortikoide sind wirksam und gut verträglich]. Therapiewoche 1996;46(24):1380.
Sandrini 2003 {published data only}
-
- Sandrini A, Plit M, Glaville A, Bryant D, Yates DH. Effect of inhaled steroid withdrawal on exhaled nitric oxide in COPD: preliminary data [Abstract]. Thorax 2003;58(Suppl 3):iii86.
Sapey 2000 {published data only}
-
- Sapey E, Langford NJ, Kendall MJ. Inhaled corticosteroids and chronic obstructive pulmonary disease. Journal of Clinical Pharmacy & Therapeutics 2000;25(4):235‐8. - PubMed
Schuurmans 2001 {published data only}
-
- Schuurmans M, Soler M. COPD ‐ Therapie: Bronchodilatatoren und Steroide. Therapiewoche 2001;17(1):28‐32.
Spicuzza 2004 {published data only}
-
- Spicuzza L, Scuderi V, Prosperini G, Balsamo R, DiMara GU, Polosa R. Acute effect of inhaled fluticasone on airway hyperresponsiveness to adenosine 5'‐monophosphate in asthma and in COPD. American Thoracic Society 100th International Conference. 2004; Vol. A58 Poster K33.
Tsang 1999 {published data only}
-
- Tsang KW. Inhaled corticosteroids in COPD. Thorax 1999;54(2):186. - PubMed
Turker 2004 {published data only}
-
- Turker H, Karakurt Z, Durucu K, Sulu E, Boga S, Yavsan M. High dose inhaler corticosteroids in patients with COPD. European Respiratory Journal 2004;24(Suppl 48):289s.
van den Boom 2001 {published data only}
-
- Boom G, Rutten‐Van Molken MPMJ, Molema J, Tirimanna PRS, Weel C, Schayck CP. The cost effectiveness of early treatment with fluticasone propionate 250 mcg twice a day in subjects with obstructive airway disease: results of the DIMCA program. American Journal of Respiratory and Critical Care Medicine 2001;164(11):2057‐66. - PubMed
van der Valk 2002 {published data only}
-
- Valk P, Monninkhof E, Phalen J, Zielhuis G, Herwaarden C. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. American Journal of Respiratory and Critical Care Medicine 2002;166(10):1358‐63. - PubMed
van Grunsven 2000 {published data only}
-
- Grunsven PM, Schayck CP, Deuveren M, Herwaarden CL, Akkermans RP, Weel C. Compliance during long‐term treatment with fluticasone propionate in subjects with early signs of asthma or chronic obstructive pulmonary disease (COPD): results of the Detection, Intervention, and Monitoring Program of COPD and Asthma (DIMCA) Study. Journal of Asthma 2000;37(3):225‐34. - PubMed
van Schayck 1995 {published data only}
-
- Schayck CP, Dompeling E, Rutten MP, Folgering H, Boom G, Weel C. The influence of an inhaled steroid on quality of life in patients with asthma or COPD. Chest 1995;107(5):1199‐205. - PubMed
Vestbo 2000 {published data only}
-
- Vestbo J, Sørensen T, Lange P, Brix A, Torre P, Viskum K. Long‐term effect of inhaled budesonide in patients with mild to moderate chronic obstructive lung disease. The Østerbro Study [Langtidsvirkningen af inhaleret budesonid hos patienter med mild og moderat kronisk obstruktiv lungesygdom. Østerbro lunge studie]. Ugeskrift for Læger 2000;162(4):493‐7. - PubMed
Watson 1992 {published data only}
-
- Watson A, Lim TW, Joyce H, Pride NB. Failure of inhaled corticosteroids to modify bronchoconstrictor or bronchodilator responsiveness in middle‐aged smokers with mild airflow obstruction. Chest 1992;101(2):350‐5. - PubMed
Weiner 1997 {published data only}
-
- Weiner P, Zamir D, Beckerman M. Inhaled budesonide for chronic obstructive pulmonary disease. Harefuah 1997;132(11):823. - PubMed
Weir 1993 {published data only}
-
- Weir DC, Burge PS. Effects of high dose inhaled beclomethasone dipropionate, 750 mcg and 1500 mcg twice daily, and 40 mg per day oral prednisolone on lung function, symptoms, and bronchial hyperresponsiveness in patients with non‐asthmatic chronic airflow obstruction. Thorax 1993;48(4):309‐16. - PMC - PubMed
Wesseling 1991 {published data only}
-
- Wesseling GJ, Quaedvlieg M, Wouters EFM. Inhaled budesonide in chronic bronchitis. Effects on respiratory impedance. European Respiratory Journal 1991;4(9):1101‐5. - PubMed
Whittaker 2000 {published data only}
-
- Whittaker AJ, Spiro SG. Inhaled steroid therapy in chronic obstructive pulmonary disease. Current Opinion in Pulmonary Medicine 2000;6(2):104‐9. - PubMed
Wilcke 1997 {published data only}
-
- Wilcke JT, Dirksen A. The effect of inhaled glucocorticosteroids in emphysema due to alpha1‐antitrypsin deficiency. Respiratory Medicine 1997;91(5):275‐9. - PubMed
Williamson 2009 {published data only}
-
- Williamson P, Menzies D, Clearie K, Vaidyanathan S, Lipworth B. Dose response for inhaled fluticasone on airway and systemic inflammation in COPD [Abstract]. European Respiratory Society Annual Congress. Vienna, Austria, September 12‐16 2009:2015. - PubMed
Yildiz 2000 {published data only}
-
- Yildiz F, Kaur AC, Ilgazli A, Celikoglu M, Kacar Ozkara S, Paksoy N, et al. Inhaled corticosteroids may reduce neutrophilic inflammation in patients with stable chronic obstructive pulmonary disease. Respiration 2000;67(1):71‐6. - PubMed
Additional references
Agarwal 2010
-
- Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Inhaled corticosteroids vs placebo for preventing COPD exacerbations: a systematic review and metaregression of randomized controlled trials. Chest. 2009/09/29 2010; Vol. 137, issue 2:318‐25. [1931‐3543: (Electronic)] - PubMed
Alsaeedi 2002
-
- Alsaeedi A, Sin DD, McAlister FA. The effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review of randomised placebo‐controlled trials. American Journal of Medicine 2002;113(1):59‐65. - PubMed
Barnes 2000
-
- Barnes PJ. Inhaled corticosteroids are not beneficial in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2000;161(2):342‐4. - PubMed
Barnes 2004
-
- Barnes PJ, Ito K, Adcock IM. Corticosteroid resistance in chronic obstructive pulmonary disease: inactivation of histone deacetylase. Lancet 2004;363(9410):731‐3. - PubMed
Bonay 2002
-
- Bonay M, Bancal C, Crestani B. Benefits and risks of inhaled corticosteroids in chronic obstructive pulmonary disease. Drug Safety 2002;25(1):57‐71. - PubMed
Bonay 2005
-
- Bonay M, Bancal C, Crestani B. The risk/benefit of inhaled corticosteroids in chronic obstructive pulmonary disease. Expert Opinion on Drug Safety 2005;4(2):251‐71. - PubMed
Burge 2001
-
- Burge S. Should inhaled corticosteroids be used in the long term treatment of chronic obstructive pulmonary disease?. Drugs 2001;61(11):1535‐44. - PubMed
Burge 2003a
Burge 2003b
Calverley 1999
Calverley 2000
-
- Calverley PM. Inhaled corticosteroids are beneficial in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2000;161(2):341‐2. - PubMed
Calverley 2003d
-
- Calverley PM, Spencer S, Willits L, Burge PS, Jones PW. Withdrawal from treatment as an outcome in the ISOLDE study of COPD. Chest 2003;124(4):1350. - PubMed
Calverley 2005
-
- Calverley PM. The role of corticosteroids in chronic obstructive pulmonary disease. Seminars in Respiratory and Critical Care Medicine 2005;26(2):235‐45. - PubMed
Calverley 2011
-
- Calverley PM, Stockley RA, Seemungal TA, Hagan G, Willits LR, Riley JH, et al. Reported pneumonia in patients with COPD: findings from the INSPIRE study. Chest. 2010/06/26 2011; Vol. 139, issue 3:505‐12. [1931‐3543: (Electronic)] - PubMed
Celli 2008
-
- Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. American Journal of Respiratory and Critical Care Medicine 2008;178(4):332‐8. - PubMed
Crim 2009
-
- Crim C, Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. European Respiratory Journal. 2009/05/16 2009; Vol. 34, issue 3:641‐7. [1399‐3003: (Electronic)] - PubMed
Donaldson 2002
Donaldson 2006
Drummond 2008
Eichenhorn 2003
-
- Eichenhorn MS, Wise RA, Madhok TC, Gerald LB, Bailey WC, Tashkin DP, et al. Lack of long‐term adverse adrenal effects from inhaled triamcinolone: Lung Health Study II. Chest 2003;124(1):57‐62. - PubMed
Epstein 2003
-
- Epstein PE. Inhaled corticosteroids and chronic obstructive pulmonary disease: are we barking up the wrong tracheobronchial tree?. Annals of Internal Medicine 2003;138(12):1001‐2. - PubMed
Ferguson 2006
-
- Ferguson GT, Calverley PMA, Anderson JA, et al. The TORCH (TOwards a Revolution in COPD Health) study: salmeterol/fluticasone propionate (SFC) improves survival in COPD over three years. European Respiratory Journal 2006;28(Suppl 50):34s.
Gan 2005
Gartlehner 2006
Gizycki 2002
Highland 2003
-
- Highland KB, Strange C, Heffner JE. Long‐term effects of inhaled corticosteroids on FEV1 in patients with chronic obstructive pulmonary disease: a meta‐analysis. Annals of Internal Medicine 2003;138(12):969‐73. - PubMed
Highland 2004
-
- Highland KB. Inhaled corticosteroids in chronic obstructive pulmonary disease: is there a long‐term benefit?. Current Opinion in Pulmonary Medicine 2004;10(2):113‐9. - PubMed
Hudson 1990
-
- Hudson LD, Monti CM. Rationale and use of corticosteroids in chronic obstructive pulmonary disease. Medical Clinics of North America 1990;74(3):661‐90. - PubMed
Ito 2005
-
- Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. New England Journal of Medicine 2005;325(19):1967‐76. - PubMed
Johnell 2002
-
- Johnell O, Pauwels R, Löfdahl CG, Laitinen LA, Postma DS, Pride NB, et al. Bone mineral density in patients with chronic obstructive pulmonary disease treated with budesonide Turbuhaler. European Respiratory Journal 2002;19(6):1058‐63. - PubMed
Jones 2003
-
- Jones PW, Willits LR, Burge PS, Calverley PMA. Disease severity and the effect of fluticasone propionate on chronic obstructive pulmonary disease exacerbations. European Respiratory Journal 2003;21(1):68‐73. - PubMed
Kanner 2001
-
- Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV1 decline in current smokers but not ex‐smokers with mild chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2001;164(3):358‐64. - PubMed
Loke 2011
-
- Loke YK, Cavallazzi R, Singh S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta‐analysis of randomised controlled trials and observational studies. Thorax. 2011/05/24 2011; Vol. 66, issue 8:699‐708. [1468‐3296: (Electronic)] - PubMed
Macie 2006
-
- Macie C, Wooldrage K, Manfreda J, Anthonisen NR. Inhaled corticosteroids and mortality in COPD. Chest 2006;130(3):640‐6. - PubMed
Man 2005a
-
- Man SF, Sin DD. Effects of corticosteroids on systemic inflammation in chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2005;2(1):78‐82. - PubMed
Man 2005b
-
- Man SF, Sin DD. Inhaled corticosteroids in chronic obstructive pulmonary disease: is there a clinical benefit?. Drugs 2005;65(5):579‐91. - PubMed
Mapel 2006
-
- Mapel DW, Hurley JS, Roblin D, Roberts M, Davis KJ, Schreiner R, et al. Survival of COPD patients using inhaled corticosteroids and long‐acting beta agonists. Respiratory Medicine 2006;100(4):595‐609. - PubMed
Mapp 2000
-
- Mapp CE. Inhaled glucocorticoids in chronic obstructive pulmonary disease. New England Journal of Medicine 2000;343(26):1960‐1. - PubMed
Postma 1999
-
- Postma DS, Kerstjens HA. Are inhaled glucocorticosteroids effective in chronic obstructive pulmonary disease?. American Journal of Respiratory and Critical Care Medicine 1999;160(5):s66‐s71. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Riancho 2002
-
- Riancho JA, Cubian I, Portero I. Effectiveness of inhaled corticosteroids in chronic obstructive lung disease: systematic review. Medica Clinica 2002;118(12):446‐51. - PubMed
Scanlon 2004
-
- Scanlon PD, Connett JE, Wise RA, Tashkin DP, Madhok T, Skeans M, et al. Loss of bone density with inhaled triamcinolone in Lung Health Study II. American Journal of Respiratory and Critical Care Medicine 2004;170(12):1302‐9. - PubMed
Scott 2006
Selroos 2004
-
- Selroos O. The place of inhaled corticosteroids in chronic obstructive pulmonary disease. Current Medical Research and Opinion 2004;20(10):1579‐93. - PubMed
Sin 2001
-
- Sin DD, Tu JV. Inhaled corticosteroids and the risk of mortality and readmission in elderly patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2001;164(4):580‐4. - PubMed
Sin 2003a
-
- Sin DD, Man SF. Inhaled corticosteroids and survival in chronic obstructive pulmonary disease: does the dose matter?. European Respiratory Journal 2003;21(2):260‐6. - PubMed
Sin 2003b
-
- Sin DD, Man SF. Inhaled corticosteroids in the long‐term management of patients with chronic obstructive pulmonary disease. Drugs & Aging 2003;20(12):867‐80. - PubMed
Sin 2003c
-
- Sin DD, McAlister FA, Man SF, Anthonisen NR. Contemporary management of chronic obstructive pulmonary disease: scientific review. JAMA 2003;290(17):2301‐12. - PubMed
Sin 2005
Sin 2009
-
- Sin DD, Tashkin D, Zhang X, Radner F, Sjobring U, Thoren A, et al. Budesonide and the risk of pneumonia: a meta‐analysis of individual patient data. Lancet. 2009/09/01 2009; Vol. 374, issue 9691:712‐9. [1474‐547X: (Electronic)] - PubMed
Sin 2010
-
- Sin DD, Man SFP. Steroids in COPD: still up in the air?. European Respiratory Journal 2010;35(4):949‐51. - PubMed
Singh 2009
-
- Singh S, Amin AV, Loke YK. Long‐term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta‐analysis. Archives of Internal Medicine. 2009/02/11 2009; Vol. 169, issue 3:219‐29. [1538‐3679: (Electronic)] - PubMed
Soriano 2003
-
- Soriano JB, Kiri VA, Pride NB, Vestbo J. Inhaled corticosteroids with/without long‐acting beta‐agonists reduce the risk of rehospitalization and death in COPD patients. American Journal of Respiratory and Critical Care Medicine 2003;2(1):67‐74. - PubMed
Soriano 2007
-
- Soriano JB, Sin DD, Zhang X, Anderson JA, Anthonisen NR, Buist AS, et al. A pooled analysis of FEV1 decline in COPD patients randomized to inhaled corticosteroids or placebo. Chest 2007;131:682‐9. - PubMed
Spencer 2001
-
- Spencer S, Calverley PM, Burge PS, Jones PW. Health status deterioration in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2001;163(1):122‐8. - PubMed
Suissa 2003
-
- Suissa S. Effectiveness of inhaled corticosteroids in chronic obstructive pulmonary disease: immortal time bias in observational studies. American Journal of Respiratory and Critical Care Medicine 2003;168(1):49‐53. - PubMed
Suissa 2004
-
- Suissa S. Inhaled steroids and mortality in COPD: bias from unaccounted immortal time. European Respiratory Journal 2004;23(3):391‐5. - PubMed
Suissa 2006
-
- Suissa S. Statistical treatment of exacerbations in therapeutic trials of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2006;173(8):842‐6. - PubMed
Suissa 2008
-
- Suissa S. Medications to modify lung function decline in chronic obstructive pulmonary disease: some hopeful signs. American Journal of Respiratory and Critical Care Medicine. 2008/08/05 2008; Vol. 178, issue 4:322‐3. [1535‐4970: (Electronic)] - PubMed
Suissa 2008a
-
- Suissa S, Ernst P, Vandemheen KL, Aaron SD. Methodological issues in therapeutic trials of COPD. European Respiratory Journal 2008;31:927‐33. - PubMed
Sutherland 2003
van Schayck 1996
-
- Schayck CP, Grunsven PM, Dekhuijzen PN. Do patients with COPD benefit from treatment with inhaled corticosteroids?. European Respiratory Journal 1996;9(10):1969‐72. - PubMed
Verhoeven 2000
Verhoeven 2001
Vestbo 2011
-
- Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. Changes in forced expiratory volume in 1 second over time in COPD. New England Journal of Medicine. 2011/10/14 2011; Vol. 365, issue 13:1184‐92. [1533‐4406: (Electronic)] - PubMed
Wedzicha 2005
Weir 1990b
Weir 1991
-
- Weir DC, Gove RI, Robertson AS, Burge PS. Response to corticosteroids in chronic airflow obstruction: relationship to emphysema and airways collapse. European Respiratory Journal 1991;4(10):1185‐90. - PubMed
Welte 2009
-
- Welte T. Inhaled corticosteroids in COPD and the risk of pneumonia. Lancet. 2009/09/01 2009; Vol. 374, issue 9691:668‐70. [1474‐547X: (Electronic)] - PubMed
Woodhead 2007
-
- Woodhead M. Inhaled corticosteroids cause pneumonia ...or do they?. American Journal of Respiratory and Critical Care Medicine. 2007/07/10 2007; Vol. 176, issue 2:111‐2. [1073‐449X: (Print)] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

