Combined oral contraceptive pills for treatment of acne
- PMID: 22786490
- PMCID: PMC11437354
- DOI: 10.1002/14651858.CD004425.pub6
Combined oral contraceptive pills for treatment of acne
Abstract
Background: Acne is a common skin disorder among women. Although no uniform approach to the management of acne exists, combination oral contraceptives (COCs), which contain an estrogen and a progestin, often are prescribed for women.
Objectives: To determine the effectiveness of combined oral contraceptives (COCs) for the treatment of facial acne compared to placebo or other active therapies.
Search methods: In January 2012, we searched for randomized controlled trials of COCs and acne in the computerized databases of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, POPLINE, and LILACS. We also searched for clinical trials in ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP) (Aug 2011). For the initial review, we wrote to researchers to seek any unpublished or published trials that we might have missed.
Selection criteria: We considered randomized controlled trials reported in any language that compared the effectiveness of a COC containing an estrogen and a progestin to placebo or another active therapy for acne in women.
Data collection and analysis: We extracted data on facial lesion counts, both total and specific (i.e., open or closed comedones, papules, pustules and nodules); acne severity grades; global assessments by the clinician or the participant, and discontinuation due to adverse events. Data were entered and analyzed in RevMan. For continuous data, we calculated the mean difference (MD) and 95% confidence interval (CI). For dichotomous data, we calculated the Peto odds ratio (OR) and 95% CI.
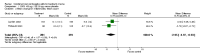
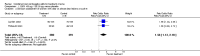
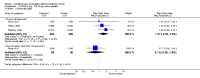
Main results: The review includes 31 trials with 12,579 participants. Of 24 comparisons made, 6 compared a COC to placebo, 17 different COCs, and 1 compared a COC to an antibiotic. Of nine placebo-controlled trials with data for analysis, all showed COCs reduced acne lesion counts, severity grades and self-assessed acne compared to placebo. A levonorgestrel-COC group had fewer total lesion counts (MD -9.98; 95% CI -16.51 to -3.45), inflammatory and non-inflammatory lesion counts, and were more likely to have a clinician assessment of clear or almost clear lesions and participant self-assessment of improved acne lesions. A norethindrone acetate COC had better results for clinician global assessment of no acne to mild acne (OR 1.86; 95% CI 1.32 to 2.62). In two combined trials, a norgestimate COC showed reduced total lesion counts (MD-9.32; 95% CI -14.19 to -4.45), reduced inflammatory lesion and comedones counts, and more with clinician assessment of improved acne. For two combined trials of a drospirenone COC, the investigators' assessment of clear or almost clear skin favored the drospirenone group (OR 3.02; 95% CI 1.99 to 4.59). In one trial, the drospirenone-COC group showed greater (more positive) percent changes for total lesion count (MD 29.08; 95% CI 3.13 to 55.03), inflammatory and non-inflammatory lesion counts, and papule and closed comedone counts. A dienogest-COC group had greater percentage decreases in total lesion count (MD -15.30; 95% CI -19.98 to -10.62) and inflammatory lesion count, and more women assessed with overall improvement of facial acne. A CMA-COC group had more 'responders,' those with 50% or greater decrease in facial papules and pustules (OR 2.31; 95% CI 1.50 to 3.55)Differences in the comparative effectiveness of COCs containing varying progestin types and dosages were less clear, and data were limited for any particular comparison. COCs that contained chlormadinone acetate or cyproterone acetate improved acne better than levonorgestrel. A COC with cyproterone acetate showed better acne outcomes than one with desogestrel, but the studies produced conflicting results. Likewise, levonorgestrel showed a slight improvement over desogestrel in acne outcomes, but results were not consistent. A drospirenone COC appeared to be more effective than norgestimate or nomegestrol acetate plus 17β-estradiol but less effective than cyproterone acetate.
Authors' conclusions: This update yielded six new trials but no change in conclusions. The six COCs evaluated in placebo-controlled trials are effective in reducing inflammatory and non-inflammatory facial acne lesions. Few important and consistent differences were found between COC types in their effectiveness for treating acne. How COCs compare to alternative acne treatments is unknown since only one trial addressed this issue. The use of standardized methods for assessing acne severity would help in synthesizing results across trials as well as aid in interpretation.
Conflict of interest statement
Dr Grimes has consulted with the pharmaceutical companies Bayer Healthcare Pharmaceuticals and Merck & Co, Inc.
Figures























































































































Update of
-
Combined oral contraceptive pills for treatment of acne.Cochrane Database Syst Rev. 2012 Jun 13;(6):CD004425. doi: 10.1002/14651858.CD004425.pub5. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2012 Jul 11;(7):CD004425. doi: 10.1002/14651858.CD004425.pub6. PMID: 22696343 Updated.
References
References to studies included in this review
Aydinlik 1986 {published data only}
-
- Aydinlik S, Lachnit‐Fixson U, Lehnert J. Reduced estrogen ovulation inhibitor in acne therapy. Double‐blind study comparing Diane‐35 to Diane [Ostrogenreduzierter ovulationshemmer zur aknetherapie.]. Fortschritte der Medizin 1986;104:547‐50. - PubMed
Bayer 2011 {published data only}
-
- Bayer Schering Pharma AG. GA YAZ ACNE in China Phase III. http://clinicaltrials.gov/ct2/show/NCT00818519 (accessed 25 Aug 2011).
Carlborg 1986 {published and unpublished data}
-
- Carlborg L. Cyproterone acetate versus levonorgestrel combined with ethinyl estradiol in the treatment of acne. Results of a multicenter study. Acta Obstetricia et Gynecologica Scandinavica 1986;134:29‐32. - PubMed
Charoenvisal 1996 {published data only}
-
- Charoenvisal C, Thaipisuttikul Y, Pinjaroen S, Krisanapan O, Benjawang W, Koster A, et al. Effects on acne of two oral contraceptives containing desogestrel and cyproterone acetate. International Journal of Fertility and Menopausal Studies 1996;41:423‐9. - PubMed
Dieben 1994 {published and unpublished data}
-
- Dieben TO, Vromans L, Theeuwes A, Bennink HJ. The effects of CTR‐24, a biphasic oral contraceptive combination, compared to Diane‐35 in women with acne. Contraception 1994;50:373‐82. - PubMed
Fugere 1988 {published data only}
-
- Fugere P, Percival‐Smith R, Lussier‐Cacan S, Tetrault C, Farquhar DJ. The comparative efficacy and safety of Diane‐35 versus Diane‐50 in the treatment of moderate to severe acne and seborrhea: 12‐month results. Recent Research on Gynecological Endocrinology 1988;1:590‐608.
-
- Fugere P, Percival‐Smith RK, Lussier‐Cacan S, Davignon J, Farquhar D. Cyproterone acetate/ethinyl estradiol in the treatment of acne. A comparative dose‐response study of the estrogen component. Contraception 1990;42:225‐34. - PubMed
Halbe 1998 {published data only}
-
- Halbe HW, Melo NR, Bahamondes L, Petracco A, Lemgruber M, Andrade RP, et al. Efficacy and acceptability of two monophasic oral contraceptives containing ethinylestradiol and either desogestrel or gestodene. European Journal of Contraception and Reproductive Health Care 1998;3:113‐20. - PubMed
J&J 2005 {published data only}
-
- Johnson, Johnson Taiwan Ltd. Comparison of efficacy and safety of norgestimate‐ethinyl estradiol and cyproterone acetate‐ethinyl estradiol in the treatment of acne vulgaris. http://clinicaltrials.gov/ct2/show/NCT00752635 (accessed 25 Aug 2011).
Kelly 2010 {published data only (unpublished sought but not used)}
-
- Kelly S, Davies E, Fearns S, McKinnon C, Carter R, Gerlinger C, et al. Effects of oral contraceptives containing ethinylestradiol with either drospirenone or levonorgestrel on various parameters associated with well‐being in healthy women: a randomized, single‐blind, parallel‐group, multicentre study. Clinical Drug Investigation 2010; Vol. 30, issue 5:325‐36. - PubMed
Koetsawang 1995 {published data only}
-
- Koetsawang S, Charoenvisal C, Banharnsupawat L, Singhakovin S, Kaewsuk O, Punnahitanont S. Multicenter trial of two monophasic oral contraceptives containing 30 mcg ethinylestradiol and either desogestrel or gestodene in Thai women. Contraception 1995;51:225‐9. - PubMed
Koltun 2008 {published data only}
-
- Koltun W, Lucky AW, Thiboutot D, Niknian M, Sampson‐Landers C, Korner P, et al. Efficacy and safety of 3 mg drospirenone/ 20 mcg ethinylestradiol oral contraceptive administered in 24/4 regimen in the treatment of acne vulgaris: a randomized, double‐blind, placebo‐controlled trial. Contraception 2008;77:249‐56. - PubMed
-
- Lucky AW, Koltun W, Thiboutot D, Niknian M, Sampson‐Landers C, Korner P, et al. A combined oral contraceptive containing 3‐mg drospirenone/ 20‐µg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double‐blind, placebo‐controlled study evaluating lesion counts and participant self‐assessment. Cutis 2008;82:143‐50. - PubMed
Lachnit‐Fixson 1977 {published data only}
-
- Lachnit‐Fixson U, Kaufmann J. [Therapy of androgenization symptoms: double blind study of an antiandrogen preparation (SH B 209 AB) against neogynon]. Medizinische Klinik 1977;72:1922‐6. - PubMed
Leyden 2002 {published data only}
-
- Leyden J, Shalita A, Hordinsky M, Swinyer L, Stanczyk FZ, Weber ME. Efficacy of a low‐dose oral contraceptive containing 20 ug of ethinyl estradiol and 100 ug of levonorgestrel for the treatment of moderate acne: a randomized, placebo‐controlled trial. Journal of the American Academy of Dermatology 2002;47:399‐409. - PubMed
Lucky 1997 {published data only}
-
- Lucky AW, Henderson TA, Olson WH, Robisch DM, Lebwohl M, Swinyer LJ. Effectiveness of norgestimate and ethinyl estradiol in treating moderate acne vulgaris. Journal of the American Academy of Dermatology 1997;37:746‐54. - PubMed
Maloney 2001 {published data only}
-
- Maloney JM, Arbit DI, Flack M, McLaughlin‐Miley C, Sevilla C, Derman R. Use of low‐dose oral contraceptive containing norethindrone acetate and ethinyl estradiol in the treatment of moderate acne vulgaris. Clinical Journal of Women's Health 2001;1:123‐31.
Maloney 2008 {published data only}
-
- Maloney JM, Dietze P, Watson D, Niknian M, Lee‐Rugh S, Sampson‐Landers C, et al. A randomized controlled trial of a low‐dose combined oral contraceptive containing 3 mg drospirenone plus 20 microg ethinylestradiol in the treatment of acne vulgaris: lesion counts, investigator ratings and subject self‐assessment. Journal of drugs in dermatology 2009, issue 9:837‐44. - PubMed
-
- Maloney JM, Dietze P, Watson D, Niknian M, Lee‐Rugh S, Sampson‐Landers C, et al. Treatment of acne using a 3‐milligram drospirenone/ 20‐microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstetrics and Gynecology 2008;112:773‐81. - PubMed
Mango 1996 {published data only}
-
- Mango D, Ricci S, Manna P, Miggiano GA, Serra GB. Clinical and hormonal effects of ethinylestradiol combined with gestodene and desogestrel in young women with acne vulgaris. Contraception 1996;53:163‐70. - PubMed
Mansour 2011 {published data only}
-
- Mansour D, Verhoeven C, Sommer W, Weisberg E, Taneepanichskul S, Melis GB, et al. Efficacy and tolerability of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β‐oestradiol in a 24/4 regimen, in comparison to an oral contraceptive containing ethinylestradiol and drospirenone in a 21/7 regimen. European Journal of Contraception and Reproductive Health Care. 2011/10/15 2011; Vol. 16, issue 6:430‐43. - PMC - PubMed
Monk 1987 {published data only}
-
- Monk BE, Almeyda JA, Caldwell IW, Green B, Pelta D, Leonard J, et al. Efficacy of low‐dose cyproterone acetate compared with minocycline in the treatment of acne vulgaris. Clinical and Experimental Dermatology 1987;12:319‐22. - PubMed
Palatsi 1984 {published and unpublished data}
-
- Palatsi R, Hirvensalo E, Liukko P, Malmiharju T, Mattila L, Riihiluoma P, et al. Serum total and unbound testosterone and sex hormone binding globulin (SHBG) in female acne patients treated with two different oral contraceptives. Acta Dermato‐Venereologica 1984;64:517‐23. - PubMed
-
- Palatsi R, Reinila M, Kivinen S. Pituitary function and DHEA‐S in male acne and DHEA‐S, prolactin and cortisol before and after oral contraceptive treatment in female acne. Acta Dermato‐Venereologica 1986;66:225‐30. - PubMed
Palombo‐Kinne 2009 {published data only}
-
- Palombo‐Kinne E, Schellschmidt I, Schumacher U, Gräser T. Efficacy of a combined oral contraceptive containing 0.030 mg ethinylestradiol/2 mg dienogest for the treatment of papulopustular acne in comparison with placebo and 0.035 mg ethinylestradiol/2 mg cyproterone acetate. Contraception 2009; Vol. 79, issue 4:282‐9. - PubMed
Plewig 2009 {published data only}
-
- Plewig G, Cunliffe WJ, Binder N, Höschen K. Efficacy of an oral contraceptive containing EE 0.03 mg and CMA 2 mg (Belara) in moderate acne resolution: a randomized, double‐blind, placebo‐controlled Phase III trial. Contraception 2009; Vol. 80, issue 1:25‐33. - PubMed
Redmond 1997 {published data only}
-
- Olson WH, Lippman JS, Robisch DM. The duration of response to norgestimate and ethinyl estradiol in the treatment of acne vulgaris. International Journal of Fertility and Women's Medicine 1998;43:286‐90. - PubMed
-
- Redmond GP, Olson WH, Lippman JS, Kafrissen ME, Jones TM, Jorizzo JL. Norgestimate and ethinyl estradiol in the treatment of acne vulgaris: a randomized, placebo‐controlled trial. Obstetrics and Gynecology 1997;89:615‐22. - PubMed
Rosen 2003 {published data only}
-
- Rosen MP, Breitkopf DM, Nagamani M. A randomized controlled trial of second‐ versus third‐generation oral contraceptives in the treatment of acne vulgaris. American Journal of Obstetrics and Gynecology 2003;188:1158‐60. - PubMed
Thiboutot 2001 {published data only}
-
- Thiboutot D, Archer DF, Lemay A, Washenik K, Roberts J, Harrison DD. A randomized, controlled trial of a low‐dose contraceptive containing 20 µg of ethinyl estradiol and 100 µg of levonorgestrel for acne treatment. Fertility and Sterility 2001;76:461‐8. - PubMed
Thorneycroft 1999 {published data only}
-
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, Ballagh SA, Nichols M, Weber ME. Effect of low‐dose oral contraceptives on androgenic markers and acne. Contraception 1999;60:255‐62. - PubMed
Thorneycroft 2004 {published data only}
-
- Thorneycroft H, Gollnick H, Schellschmidt I. Superiority of a combined contraceptive containing drospirenone to a triphasic preparation containing norgestimate in acne treatment. Cutis 2004;74:123‐30. - PubMed
Van Vloten 2002 {published data only}
-
- Vloten WA, Haselen CW, Zuuren EJ, Gerlinger C, Heithecker R. The effect of 2 combined oral contraceptives containing either drospirenone or cyproterone acetate on acne and seborrhea. Cutis 2002;69 Suppl (4):2‐15. - PubMed
Vartiainen 2001 {published data only}
-
- Vartiainen M, Gezelle H, Broekmeulen CJ. Comparison of the effect on acne with a combiphasic desogestrel‐containing oral contraceptive and a preparation containing cyproterone acetate. European Journal of Contraception and Reproductive Health Care 2001;6:46‐53. - PubMed
Winkler 2004 {published and unpublished data}
-
- Winkler UH, Ferguson H, Mulders JAPA. Cycle control, quality of life and acne with two low‐dose oral contraceptives containing 20 µg ethinylestradiol. Contraception 2004;69:469‐76. - PubMed
Worret 2001 {published data only}
-
- Worret I, Arp W, Zahradnik HP, Andreas JO, Binder N. Acne resolution rates: results of a single‐blind, randomized, controlled, parallel phase III trial with EE/CMA (Belara) and EE/LNG (Microgynon). Dermatology 2001;203:38‐44. - PubMed
References to studies excluded from this review
Barranco 1974 {published data only}
-
- Barranco VP. Effect of androgen‐dominant and estrogen‐dominant oral contraceptives on acne. Cutis 1974;4:384‐6.
Carmina 2002 {published data only}
-
- Carmina E, Godwin AJ, Stanczyk FZ, Lippman JS, Lobo RA. The association of serum androsterone glucuronide with inflammatory lesions in women with adult acne. Journal of Endocrinological Investigation 2002;25:765‐8. - PubMed
Chapdelaine 1989 {published data only}
-
- Chapdelaine A, Desmarais JL, Derman RJ. Clinical evidence of minimal androgenic activity of norgestimate. International Journal of Fertility 1989;34:347‐52. - PubMed
Coney 2001 {published data only}
-
- Coney P, Washenik K, Langley RGB, DiGiovanna JJ, Harrison DD. Weight change and adverse event incidence with a low dose contraceptive: two randomized, placebo‐controlled trials. Contraception 2001;63:297‐302. - PubMed
Cremoncini 1976 {published data only}
-
- Cremoncini C, Vignati E, Libroia A. Treatment of hirsutism and acne in women with two combinations of cyproterone acetate and ethinylestradiol [Trattamento dell'irsutismo e dell'acne nella donna con due associazioni di ciproterone acetato ed etinil‐estradiolo]. Acta Europaea Fertilitatis 1976;7:299‐314. - PubMed
Erdmann 1994 {published data only}
-
- Erdmann D, Schindler EM, Schindler AE. Ovarian suppression with Diane 35/50 [Die ovarielle Suppression unter Diane 35/50]. Geburtshilfe und Frauenheilkunde 1994;54:627‐33. - PubMed
Erkkola 1990 {published data only}
-
- Erkkola R, Hirvonen E, Luikku J, Lumme R, Mannikko H, Aydinlik S. Ovulation inhibitors containing cyproterone acetate or desogestrel in the treatment of hyperandrogenic symptoms. Acta Obstetricia et Gynecologica Scandinavica 1990;69:61‐5. - PubMed
Greenwood 1985 {published data only}
Gruber 1998 {published data only}
-
- Gruber DM, Sator MO, Joura EA, Kokoschka EM, Heinze G, Huber JC. Topical cyproterone acetate treatment in women with acne: a placebo‐controlled trial. Archives of Dermatology 1998;134:459‐63. - PubMed
Grund 1975 {published data only}
-
- Grund E, Schmidt‐Elmendorff H. The treatment of virilizing syndromes. Comparative clinical studies of 2 antiandrogen‐active gestagens (cyproterone acetate, megestrol acetate) [Behandlung von Virilisierungs‐erscheinungen. Vergleichende klinische Untersuchung zweier antiandrogenwirksamer Gestagene (Cyproteronazetat, Megestrolazetat)]. Die Medizinische Welt 1975;26:2180‐7. - PubMed
Huber 2000 {published data only}
-
- Huber J, Foidart JM, Wuttke W, Merki‐Feld GS, The HS, Gerlinger C, et al. Efficacy and tolerability of a monophasic oral contraceptive containing ethinylestradiol and drospirenone. European Journal of Contraception and Reproductive Health Care 2000;5:25‐34. - PubMed
Katz 2000 {published data only}
-
- Katz HI, Kempers S, Akin MD, Dunlap F, Whiting D, Norbart TC. Effect of a desogestrel‐containing oral contraceptive on the skin. European Journal of Contraception and Reproductive Health Care 2000;5:248‐55. - PubMed
Lachnit‐Fixson 1979 {published data only}
-
- Lachnit‐Fixson U. The development and evaluation of an ovulation inhibitor (DIAne) containing an antiandrogen. Acta Obstetricia et Gynecologica Scandinavica. Supplement 1979;88:33‐42. - PubMed
Mehrl 1988 {published data only}
-
- Mehrl J. Acne: long‐term therapy with side effect. Sebaceous gland activity is androgen dependent‐‐acne therapeutic drug is effective as a contraceptive [Akne: Langzeittherapie mit Nebeneffekt. Talgdrüsenaktivität androgenabhängig ‐ Aknetherapeutikum auch kontrazeptiv wirksam]. Fortschritte der Medizin 1988;106:50‐1. - PubMed
Miller 1986 {published data only}
-
- Miller JA, Wojnarowska FT, Dowd PM, Ashton RE, O'Brien TJ, Griffiths WA, et al. Anti‐androgen treatment in women with acne: a controlled trial. British Journal of Dermatology 1986;114:705‐16. - PubMed
Nilsson 1967 {published data only}
-
- Nilsson L, Solvell L. Clinical studies on oral contraceptives‐‐a randomized, doubleblind, crossover study of 4 different preparations (Anovlar(R) mite, Lyndiol(R) mite, Ovulen(R), and Volidan). Acta Obstetricia et Gynecologica Scandinavica 1967;46: Suppl (8):1‐31. - PubMed
Perrone 1987 {published data only}
-
- Perrone G, Calzolari E, Mancone M, Masci A, Steffe M, Tesseri E. Oral contraceptives and their minor side effects: comparison between three low‐dose estroprogestinic associations [Contraccettivi orali ed effeti collatereali minori: Confronto fra tre estropogestinici a basso dosaggio]. Patologia e Clinica Ostetrica e Ginecologica 1987;15:6‐11. - PubMed
Sanam 2011 {published data only}
-
- Sanam M, Ziba O. Desogestrel+ethinylestradiol versus levonorgestrel+ethinylestradiol. Which one has better affect on acne, hirsutism, and weight change. Saudi Medical Journal. 2011/01/08 2011; Vol. 32, issue 1:23‐6. - PubMed
Sanhueza 1979 {published data only}
-
- Sanhueza H, Sivin I, Kumar S, Kessler M, Carrasco A, Yee J. A randomized double blind study of two oral contraceptives. Contraception 1979;20:29‐48. - PubMed
Spona 1996 {published data only}
-
- Spona J, Elstein M, Feichtinger W, Sullivan H, Ludicke F, Muller U, et al. Shorter pill‐free interval in combined oral contraceptives decreases follicular development. Contraception 1996;54:71‐7. - PubMed
Vegetti 1996 {published data only}
-
- Vegetti W, Testa G, Maggioni P, Motta T, Falsetti L, Crosignani PG. An open randomized comparative study of an oral contraceptive containing ethinyl estradiol and cyproterone acetate with and without the GnRH analogue goserelin in the long‐term treatment of hirsutism. Gynecologic and Obstetric Investigation 1966;41:260‐8. - PubMed
Vermeulen 1988 {published data only}
-
- Vermeulen A, Rubens R. Effects of cyproterone acetate plus ethinylestradiol low dose on plasma androgens and lipids in mildly hirsute or acneic young women. Contraception 1988;38:419‐28. - PubMed
Vexiau 2002 {published data only}
-
- Vexiau P, Chaspoux C, Boubou P, Fiet J, Jouanique C, Hardy N, et al. Effect of minoxidil 2% vs. cyproterone acetate treatment on female androgenetic alopecia: a controlled, 12‐month randomized trial. British Journal of Dermatology 2002;146:992‐9. - PubMed
Volpe 1994 {published data only}
-
- Volpe A, Silferi M, Mauri A, Deiana P, Angioni S, Grasso A, et al. Efficacy on hyperandrogenism and safety of a new oral contraceptive biphasic formulation containing desogestrel. European Journal of Obstetrics and Gynecology and Reproductive Biology 1994;53:205‐9. - PubMed
Weber‐Diehl 1993 {published data only}
-
- Weber‐Diehl F, Lehnert J, Lachnit U. Comparison of two triphasic oral contraceptives containing either gestodene or norethindrone: a randomized, controlled trial. Contraception 1993;48:291‐301. - PubMed
Wendler 1995 {published data only}
-
- Wendler J, Siegert C, Schelhorn P, Klinger G, Gurr S, Kaufmann J, et al. The influence of Microgynon and Diane‐35, two sub‐fifty ovulation inhibitors, on voice function in women. Contraception 1995;52:343‐8. - PubMed
Wishart 1991 {published data only}
-
- Wishart JM. An open study of Triphasil and Diane 50 in the treatment of acne. Australasian Journal of Dermatology 1991;32:51‐4. - PubMed
References to ongoing studies
Kimball 2011 {published data only}
-
- Kimball AB, Alora‐Palli MB. A study to examine the safety and efficacy of drospirenone and ethinyl estradiol (YAZ) compared with placebo In the treatment of moderate truncal acne vulgaris. http://clinicaltrials.gov/ct2/show/NCT00722761 (accessed 25 Aug 2011). - PubMed
Additional references
Baldwin 2002
-
- Baldwin HE. The interaction between acne vulgaris and the psyche. Cutis 2002;70:133‐9. - PubMed
Bergfeld 1995
-
- Bergfeld WF. The evaluation and management of acne: economic considerations. Journal of American Academy of Dermatology. 1995;32 Suppl (5 Pt 3):52‐6. - PubMed
Burkhart 2000
-
- Burkhart CG, Butcher C, Burkhart CN, Lehmann P. Effects of benzoyl peroxide on lipogenesis in sebaceous glands using animal model. Journal of Cutaneous Medicine and Surgery 2000;4:138‐41. - PubMed
Cassidenti 1991
-
- Cassidenti DL, Paulson RJ, Serafini P, Stanczyk FZ, Lobo RA. Effects of sex steroids on skin 5 alpha‐reductase activity in vitro. Obstetrics and Gynecology 1991;78:103‐7. - PubMed
CONSORT 2009
-
- CONSORT group. CONSORT: Transparent reporting of trials. http://www.consort‐statement.org/ (accessed 15 Jul 2009).
Downie 2002
-
- Downie MM, Sanders DA, Kealey T. Modelling the remission of individual acne lesion in vitro. British Journal of Dermatology 2002;147:869‐78. - PubMed
Eady 1994
-
- Eady EA, Farmery MR, Ross JI, Cove JH, Cunliffe WJ. Effects of benzoyl peroxide and erythromycin alone and in combination against antibiotic‐sensitive and ‐resistant skin bacteria from acne patients. British Journal of Dermatology 1994;131:331‐6. - PubMed
Fotherby 1994
-
- Fotherby K, Caldwell AD. New progestogens in oral contraception. Contraception 1994;49:1‐32. - PubMed
George 2008
-
- George R, Clarke S, Thiboutot D. Hormonal therapy for acne. Seminars in Cutaneous Medicine and Surgery 2008;27:188‐96. - PubMed
Goulden 1999
-
- Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. Journal of the American Academy of Dermatology 1999;41:577‐80. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1[updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. John Wiley & Sons, Ltd, (accessed 04 Oct 2011).
Jemec 2002
-
- Jemec GB, Linneberg A, Nielsen NH, Frolund L, Madsen F, Jorgensen T. Have oral contraceptives reduced the prevalence of acne? A population‐based study of acne vulgaris, tobacco smoking and oral contraceptives. Dermatology 2002;204:179‐84. - PubMed
Koltun 2011
-
- Koltun W, Maloney JM, Marr J, Kunz M. Treatment of moderate acne vulgaris using a combined oral contraceptive containing ethinylestradiol 20 mug plus drospirenone 3mg administered in a 24/4 regimen: a pooled analysis. European Journal of Obstetrics & Gynecology and Reproductive Biology 2011;155(2):171‐5. - PubMed
Lexchin 2003
Leyden 1997
-
- Leyden JJ. Therapy for acne vulgaris. New England Journal of Medicine 1997;336:1156‐62. - PubMed
O'Connell 2008
-
- O'Connell K, Westhoff C. Pharmacology of hormonal contraceptives and acne. Cutis 2008;81(1 Suppl):8‐12. - PubMed
Poli 2001
-
- Poli F, Dreno B, Verschoore M. An epidemiological study of acne in female adults: results of a survey conducted in France. Journal of the European Academy of Dermatology and Venereology 2001;15:541‐5. - PubMed
Rabe 2000
-
- Rabe T, Kowald A, Ortmann J, Rehberger‐Schneider S. Inhibition of skin 5 alpha‐reductase by oral contraceptive progestins in vitro. Gynecological Endocrinology 2000;14:223‐30. - PubMed
Rich 2008
-
- Rich p. Hormonal contraceptives for acne management. Cutis 2008;81:13‐8. - PubMed
Schulz 2002a
-
- Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet 2002;359:614‐8. - PubMed
Schulz 2002b
-
- Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet 2002;359:696‐700. - PubMed
Seaman 2003
-
- Seaman HE, Vries CS, Farmer RD. Differences in the use of combined contraceptives amongst women with and without acne. Human Reproduction 2003;18:515‐21. - PubMed
Thiboutot 2000
-
- Thiboutot D. New treatments and therapeutic strategies for acne. Archives of Family Medicine 2000;9:179‐87. - PubMed
Toyoda 2001
-
- Toyoda M, Morohashi M. Pathogenesis of acne. Medical Electron Microscopy 2001;34:29‐40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

