First-line chemotherapy in low-risk gestational trophoblastic neoplasia
- PMID: 22786502
- PMCID: PMC4164471
- DOI: 10.1002/14651858.CD007102.pub3
First-line chemotherapy in low-risk gestational trophoblastic neoplasia
Update in
-
First-line chemotherapy in low-risk gestational trophoblastic neoplasia.Cochrane Database Syst Rev. 2016 Jun 9;2016(6):CD007102. doi: 10.1002/14651858.CD007102.pub4. Cochrane Database Syst Rev. 2016. PMID: 27281496 Free PMC article.
Abstract
Background: This is an update of a Cochrane review that was first published in Issue 1, 2009. Gestational trophoblastic neoplasia (GTN) is a rare but curable disease arising in the fetal chorion during pregnancy. Most women with low-risk GTN will be cured by evacuation of the uterus with or without single-agent chemotherapy. However, chemotherapy regimens vary between treatment centres worldwide and the comparable benefits and risks of these different regimens are unclear.
Objectives: To determine the efficacy and safety of first-line chemotherapy in the treatment of low-risk GTN.
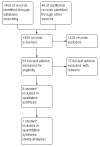
Search methods: In September 2008, we electronically searched the Cochrane Gynaecological Cancer Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL Issue 3, 2008), MEDLINE and EMBASE. In addition, we searched online trial registers, conference proceedings and reference lists of identified studies. We re-ran these searches in February 2012 for this updated review.
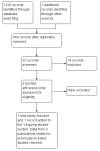
Selection criteria: For the original review, we included randomised controlled trials (RCTs), quasi-RCTs and non-RCTs that compared first-line chemotherapy for the treatment of low-risk GTN. For this updated version of the review, we included only RCTs.
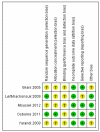
Data collection and analysis: Two review authors independently assessed studies for inclusion and extracted data to a pre-designed data extraction form. Meta-analysis was performed by pooling the risk ratio (RR) of individual trials.
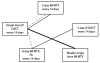
Main results: We included five moderate to high quality RCTs (517 women) in the updated review. These studies all compared methotrexate with dactinomycin. Three studies compared weekly intramuscular (IM) methotrexate with bi-weekly pulsed intravenous (IV) dactinomycin (393 women), one study compared five-day IM methotrexate with bi-weekly pulsed IV dactinomycin (75 women) and one study compared eight-day IM methotrexate-folinic acid (MTX-FA) with five-day IV dactinomycin (49 women).Overall, dactinomycin was associated with significantly higher rates of primary cure than methotrexate (five studies, 513 women; RR 0.64, 95% Confidence Interval (CI) 0.54 to 0.76). Methotrexate was associated with significantly more treatment failure than dactinomycin (five studies, 513 women; RR 3.81, 95% CI 1.64 to 8.86). We consider this evidence to be of a moderate quality.There was no significant difference between the two groups with respect to nausea (four studies, 466 women; RR 0.61, 95% CI 0.29 to 1.26) or any of the other individual side-effects reported, although data for all of these outcomes were insufficient and too heterogeneous to be conclusive. No severe adverse effects (SAEs) occurred in either group in three out of the five included studies and there was no significant difference in SAEs between the groups overall (five studies, 515 women; RR 0.35, 95% CI 0.08 to 1.66; I² = 60%), however, there was a trend towards fewer SAEs in the methotrexate group. We considered this evidence to be of a low quality due to substantial heterogeneity and low consistency in the occurrence/reporting of SAEs between trials.
Authors' conclusions: Dactinomycin is more likely to achieve a primary cure in women with low-risk GTN, and less likely to result in treatment failure, compared with methotrexate. There is limited evidence relating to side-effects, however, the pulsed dactinomycin regimen does not appear to be associated with significantly more side-effects than the low-dose methotrexate regimen and therefore should compare favourably to the five- and eight-day methotrexate regimens in this regard.We consider pulsed dactinomycin to have a better cure rate than, and a side-effect profile at least equivalent to, methotrexate when used for first-line treatment of low-risk GTN. Data from a large ongoing trial of pulsed dactinomycin compared with five- and eight-day methotrexate regimens is likely to have an important impact on our confidence in these findings.
Figures
Update of
-
First line chemotherapy in low risk gestational trophoblastic neoplasia.Cochrane Database Syst Rev. 2009 Jan 21;(1):CD007102. doi: 10.1002/14651858.CD007102.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2012 Jul 11;(7):CD007102. doi: 10.1002/14651858.CD007102.pub3. PMID: 19160319 Updated.
Similar articles
-
First-line chemotherapy in low-risk gestational trophoblastic neoplasia.Cochrane Database Syst Rev. 2016 Jun 9;2016(6):CD007102. doi: 10.1002/14651858.CD007102.pub4. Cochrane Database Syst Rev. 2016. PMID: 27281496 Free PMC article.
-
First line chemotherapy in low risk gestational trophoblastic neoplasia.Cochrane Database Syst Rev. 2009 Jan 21;(1):CD007102. doi: 10.1002/14651858.CD007102.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2012 Jul 11;(7):CD007102. doi: 10.1002/14651858.CD007102.pub3. PMID: 19160319 Updated.
-
Prophylactic chemotherapy for hydatidiform mole to prevent gestational trophoblastic neoplasia.Cochrane Database Syst Rev. 2017 Sep 11;9(9):CD007289. doi: 10.1002/14651858.CD007289.pub3. Cochrane Database Syst Rev. 2017. PMID: 28892119 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Gestational trophoblastic tumours: an update for 2014.Curr Oncol Rep. 2014 Nov;16(11):408. doi: 10.1007/s11912-014-0408-y. Curr Oncol Rep. 2014. PMID: 25318458 Review.
-
Gestational Trophoblastic Neoplasia Treatment at the Butaro Cancer Center of Excellence in Rwanda.J Glob Oncol. 2016 Apr 13;2(6):365-374. doi: 10.1200/JGO.2015.002568. eCollection 2016 Dec. J Glob Oncol. 2016. PMID: 28717722 Free PMC article.
-
Pure nongestational uterine choriocarcinoma in postmenopausal women: a case report with literature review.Cancer Biol Ther. 2019;20(9):1176-1182. doi: 10.1080/15384047.2019.1617564. Epub 2019 May 27. Cancer Biol Ther. 2019. PMID: 31132027 Free PMC article. Review.
-
Diagnosis and management of gestational trophoblastic disease: 2021 update.Int J Gynaecol Obstet. 2021 Oct;155 Suppl 1(Suppl 1):86-93. doi: 10.1002/ijgo.13877. Int J Gynaecol Obstet. 2021. PMID: 34669197 Free PMC article.
-
Prophylactic chemotherapy for hydatidiform mole to prevent gestational trophoblastic neoplasia.Cochrane Database Syst Rev. 2012 Oct 17;10(10):CD007289. doi: 10.1002/14651858.CD007289.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2017 Sep 11;9:CD007289. doi: 10.1002/14651858.CD007289.pub3. PMID: 23076934 Free PMC article. Updated.
References
References to studies included in this review
-
- Gilani MM, Yarandi F, Eftekhar Z, Hanjani P. Comparison of pulse methotrexate and pulse dactinomycin in the treatment of low-risk gestational trophoblastic neoplasia. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2005;Vol. 45(issue 2):161–4. - PubMed
-
- Lertkhachonsuk A, Tangtrakul S, Israngura N, Wilailak S. Actinomycin D versus methotrexate-folinic acid as the treatment of stage 1, low-risk gestational trophoblastic neoplasia. Conference abstract from the International Society for the Study of Trophoblastic Disease (ISSTD) conference; 2007. - PubMed
-
*
- Lertkhachonsuk AA, Israngura N, Wilailak S, Tangtrakul S. Actinomycin d versus methotrexate-folinic acid as the treatment of stage I, low-risk gestational trophoblastic neoplasia: a randomized controlled trial. International Journal of Gynecological Cancer. 2009;19(5):985–8. - PubMed
-
- Mousavi A, Cheraghi F, Yarandi F, Gilani MM, Shojaei H. Comparison of pulsed actinomycin D versus 5-day methotrexate for the treatment of low-risk gestational trophoblastic disease. International Journal of Gynecology and Obstetrics. 2012;116(1):39–42. - PubMed
-
- Osborne R, Filiaci V, Schink J, Mannel R, Provencher D, Alvarez-Secord A, et al. A randomized phase III trial comparing weekly parenteral methotrexate and “pulsed” dactinomycin as primary management for low-risk gestational trophoblastic neoplasia: A Gynecologic Oncology Group study. Gynecologic Oncology. 2008;Vol. 108:S2–S31. - PMC - PubMed
-
*
- Osborne RJ, Filiaci V, Schink JC, Mannel RS, Alvarez Secord A, Kelley JL, et al. Phase III trial of weekly methotrexate or pulsed dactinomycin for low-risk gestational trophoblastic neoplasia: a Gynecologic Oncology Group study. Journal of Clinical Oncology. 2011;29(7):825–31. - PMC - PubMed
-
- Rahimi-Moghaddam P, Eftekhar Z, Yarandi F. Single-agent therapy for low risk gestational trophoblastic tumor: a comparison between pulse-methotrexate versus pulse-actinomycin [abstract] International Journal of Gynecological Cancer. 2004;14(Suppl 1):96.
-
*
- Yarandi F, Eftekhar Z, Shojaei H, Kanani S, Sharifi A, Hanjani P. Pulse methotrexate versus pulse actinomycin D in the treatment of low-risk gestational trophoblastic neoplasia. International Journal of Gynecology and Obstetrics. 2008;Vol. 103(issue 1):33–7. - PubMed
References to studies excluded from this review
-
- Abrao RA, de Andrade JM, Tiezzi DG, Marana HR, Candido dos Reis FJ, Clagnan WS. Treatment for low-risk gestational trophoblastic disease: comparison of single-agent methotrexate, dactinomycin and combination regimens. Gynecologic Oncology. 2008;Vol. 108(issue 1):149–53. - PubMed
-
- Berkowitz RS, Goldstein DP. Methotrexate with citrovorum factor rescue for nonmetastatic gestational trophoblastic neoplasms. Obstetrics and Gynecology. 1979;Vol. 54(issue 6):725–8. - PubMed
-
- Gleeson NC, Finan MA, Fiorica JV, Robert WS, Hoffman MS, Wilson J. Nonmetastatic gestational trophoblastic disease. Weekly methotrexate compared with 8-day methotrexate-folinic acid. European Journal of Gynaecological Oncology. 1993;Vol. 14(issue 6):461–5. - PubMed
-
- Kohorn EI. Decision making for chemotherapy administration in patients with low risk gestational trophoblastic neoplasia. International Journal of Gynecological Cancer. 1996;6:279–85.
-
- Matsui H, Iitsuka Y, Seki K, Sekiya S. Comparison of chemotherapies with methotrexate, VP-16 and actinomycin-D in low-risk gestational trophoblastic disease. Remission rates and drug toxicities. Gynecologic and Obstetric Investigation. 1998;Vol. 46(issue 1):5–8. - PubMed
References to ongoing studies
-
- Schink JC, DiSaia PJ, the Gynecologic Oncology Group [14 Feb, 2012];Methotrexate or dactinomycin in treating patients with low-risk gestational trophoblastic neoplasia. www.clinicaltrials.gov/ct2/show/NCT01535053.
Additional references
-
- Aghajanian C. Treatment of low-risk gestational trophoblastic neoplasia. Journal of Clinical Oncology. 2011;29(7):786–8. - PubMed
-
- Alazzam M, Young T, Coleman R, Hancock B, Drew D, Wilson P, et al. Predicting gestational trophoblastic neoplasia (GTN): is urine hCG the answer? Gynecologic Oncology. 2011;122(3):595–9. - PubMed
-
- Andrijono A, Muhilal M. Prevention of post-mole malignant trophoblastic disease with vitamin A. Asian Pacific Journal of Cancer Prevention. 2010;11(2):567–70. - PubMed
-
- Bagshawe KD. Risk and prognostic factors in trophoblastic neoplasia. Cancer. 1976;38(3):1373–85. - PubMed
-
- Bagshawe KD, Dent J, Newlands ES, Begent RH, Rustin GJ. The role of low-dose methotrexate and folinic acid in gestational trophoblastic tumours (GTT) British Journal of Obstetrics and Gynaecology. 1989;96(7):795–802. - PubMed
References to other published versions of this review
-
- Alazzam M, Tidy J, Hancock B, Osborne R. First-line chemotherapy in low risk gestational trophoblastic neoplasia. Cochrane Database of Systematic Reviews. 2009;(Issue 1) [DOI: 10.1002/14651858.CD007102.pub2] - PubMed
-
-
* Indicates the major publication for the study
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

