Antiretroviral pre-exposure prophylaxis (PrEP) for preventing HIV in high-risk individuals
- PMID: 22786505
- PMCID: PMC11324012
- DOI: 10.1002/14651858.CD007189.pub3
Antiretroviral pre-exposure prophylaxis (PrEP) for preventing HIV in high-risk individuals
Abstract
Background: More than 30 years into the global HIV/AIDS epidemic, infection rates remain alarmingly high, with over 2.7 million people becoming infected every year. There is a need for HIV prevention strategies that are more effective. Oral antiretroviral pre-exposure prophylaxis (PrEP) in high-risk individuals may be a reliable tool in preventing the transmission of HIV.
Objectives: To evaluate the effects of oral antiretroviral chemoprophylaxis in preventing HIV infection in HIV-uninfected high-risk individuals.
Search methods: We revised the search strategy from the previous version of the review and conducted an updated search of MEDLINE, the Cochrane Central Register of Controlled Trials and EMBASE in April 2012. We also searched the WHO International Clinical Trials Registry Platform and ClinicalTrials.gov for ongoing trials.
Selection criteria: Randomised controlled trials that evaluated the effects of any antiretroviral agent or combination of antiretroviral agents in preventing HIV infection in high-risk individuals
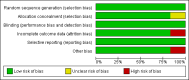
Data collection and analysis: Data concerning outcomes, details of the interventions, and other study characteristics were extracted by two independent authors using a standardized data extraction form. Relative risk with a 95% confidence interval (CI) was used as the measure of effect.
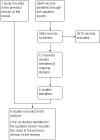
Main results: We identified 12 randomised controlled trials that meet the criteria for the review. Six were ongoing trials, four had been completed and two had been terminated early. Six studies with a total of 9849 participants provided data for this review. The trials evaluated the following: daily oral tenofovir disoproxil fumarate (TDF) plus emtricitabine (FTC) versus placebo; TDF versus placebo and daily TDF-FTC versus intermittent TDF-FTC. One of the trials had three study arms: TDF, TDF-FTC and placebo arm. The studies were carried out amongst different risk groups, including HIV-uninfected men who have sex with men, serodiscordant couples and other high risk men and women.Overall results from the four trials that compared TDF-FTC versus placebo showed a reduction in the risk of acquiring HIV infection (RR 0.51; 95% CI 0.30 to 0.86; 8918 participants). Similarly, the overall results of the studies that compared TDF only versus placebo showed a significant reduction in the risk of acquiring HIV infection (RR 0.38; 95% CI 0.23 to 0.63, 4027 participants). There were no significant differences in the risk of adverse events across all the studies that reported on adverse events. Also, adherence and sexual behaviours were similar in both the intervention and control groups.
Authors' conclusions: Finding from this review suggests that pre-exposure prophylaxis with TDF alone or TDF-FTC reduces the risk of acquiring HIV in high-risk individuals including people in serodiscordant relationships, men who have sex with men and other high risk men and women.
Conflict of interest statement
None known
Figures





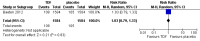







Update of
-
Antiretroviral pre-exposure prophylaxis (PrEP) for preventing HIV in high-risk individuals.Cochrane Database Syst Rev. 2009 Jan 21;(1):CD007189. doi: 10.1002/14651858.CD007189.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2012 Jul 11;(7):CD007189. doi: 10.1002/14651858.CD007189.pub3. PMID: 19160329 Updated.
Comment in
-
Antiretroviral preexposure prophylaxis for preventing HIV infection in high-risk individuals.Am Fam Physician. 2013 Aug 1;88(3):172-3. Am Fam Physician. 2013. PMID: 23939695 No abstract available.
References
References to studies included in this review
Baeten 2012 {published and unpublished data}
Grant 2010 {published data only}
Mutua 2010 {published and unpublished data}
-
- Mutua G, Sanders EJ, Kamali E, Kibengo F, Mugo P, Anzala O et a. Safety and adherence to intermittent Emtricitabine/Tenofovirfor HIV pre‐exposure prophylaxis (PrEP) in Kenya and Uganda. AIDS 2010: XVIII International AIDS Conference, Vienna, Austria. 2010:Abstract no. MOPE0369 .
Peterson 2007 {published data only}
Thigpen 2012 {published and unpublished data}
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England Journal of Medicine July 2012;367(5):423‐34. - PubMed
References to studies excluded from this review
Brooks 2003 {published data only}
-
- Brooks JJ, Scott B, Estelle P, Linda A, Charles R, Craig H, et al. A phase I/II study of nevirapine for pre‐exposure prophylaxis of HIV‐1 transmission in uninfected subjects at high risk. AIDS March 2003;17(4):547‐553. - PubMed
References to ongoing studies
Chirenje 2012 {unpublished data only}
-
- Chirenje ZM, Marrazzo J. Safety and Effectiveness of Tenofovir 1% Gel, Tenofovir Disproxil Fumarate, and Emtricitabine/Tenofovir Disoproxil Fumarate Tablets in Preventing HIV in Women. http://clinicaltrials.gov/ct2/show/NCT00705679.
Choopanya 2010 {unpublished data only}
-
- Choopanya K, Martin MT, Paxton Lynn. Bangkok Tenofovir Study. http://clinicaltrials.gov/ct2/show/NCT00119106 (Accessed 20 April 2012).
Grant 2012 {unpublished data only}
-
- Grant RM. Use of Emtricitabine and Tenofovir Disoproxil Fumarate for Pre‐Exposure Prophylaxis (ADAPT). http://clinicaltrials.gov/ct2/show/NCT01327651 (Accessed 20 April 2012).
Molina 2012 {published data only}
-
- Molina J. On Demand Antiretroviral Pre‐exposure Prophylaxis for HIV Infection in Men Who Have Sex With Men (IPERGAY). http://clinicaltrials.gov/ct2/show/NCT01473472 (Accessed 20 April 2012). [NCT01473472]
NIAID 2012 {unpublished data only}
-
- NIAID. http://clinicaltrials.gov/ct2/show/NCT01505114?term=HPTN+069&rank=1 [Evaluating the Safety and Tolerability of Antiretroviral Drug Regimens Used as Pre‐Exposure Prophylaxis to Prevent HIV Infection in Men Who Have Sex With Men (HPTN 069)]. http://clinicaltrials.gov/ct2/show/NCT01505114 (Accessed 20 April 2012).
Paxton 2007 {unpublished data only}
-
- Paxton LA. Botswana TDF/FTC Oral HIV Prophylaxis Trial. http://clinicaltrials.gov/ct2/show/NCT00448669 (Accessed 20 April 2012).
Additional references
Abdool Karim 2010
AFAO 2005
-
- Australian federation of AIDS organization Inc. AFAO National forumon Prepexposure prophylaxis for HIV(PrEP): final report. A summary of the outcomes of the National forum held at Dockside, Cockle Bay on the 16th June, 2005.
AIDS 2005
-
- AIDS News Release. Prevention in a tablet. www.ippf.org/NR/rdonlyres/80E7C1E6‐2A56‐4E18‐ACEE‐2ED784FD1FE5/0/Issue4_... (accessed 14 September 2007).
Anglemyer 2011
-
- Anglemyer A, Rutherford GW, Baggaley RC, Egger M, Siegfried N. Antiretroviral therapy for prevention of HIV transmission in HIV‐discordant couples. Cochrane Database of Systematic Reviews 2011, (8):CD009153. [PUBMED: 21833973] - PubMed
Bahuguna 2006
-
- Bahuguna NJ. Putting women in charge. www.boloji.com/wfs5/wfs672.htm (accessed 14 September 2007).
Cohen 2011
Denton 2008
Derdelinckx 2006
Desai 2008
-
- Desai K, Sansom SL, Ackers ML, Stewart SR, Hall HI, Hu DJ, et al. Modeling the impact of HIV chemoprophylaxis strategies among men who have sex with men in the United States: HIV infections prevented and cost‐effectiveness. AIDS September 2008;22(14):1829‐39. - PubMed
Donnell 2010
García‐Lerma 2008
Guay 1999
-
- Guay L, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single‐dose nevirapine compared with zidovudine for prevention of mother‐to‐child transmission of HIV‐1 in Kampala, Uganda: HIVNET 012 randomized trial. Lancet 1999;354:795‐802. - PubMed
Haase 2010
-
- Haase AT. Targeting early infection to prevent HIV‐1mucosal transmission. Nature March 2010;11(464(7286)):217‐23.. - PubMed
Highleyman 2006
-
- Highleyman L. Pre‐exposure prophylaxis: a new focus for HIV prevention. AIDS 2006 16th International AIDS Conference; 2006 Aug 13‐18; Toronto,Canada. 2006.
Pretorius 2010
Sharifi‐Azad 2011
-
- Sharifi‐Azad J, Rizzolo D. Postexposure prophylaxis for HIV: pivotal intervention for those at risk. JAAPA August 2011;24(8):22‐5. - PubMed
Siegfried 2011
-
- Siegfried N, Merwe L, Brocklehurst P, Sint TT. Antiretrovirals for reducing the risk of mother‐to‐child transmission of HIV infection. Cochrane Database of Systematic Reviews 2011, (7):CD003510. [PUBMED: 21735394] - PubMed
Smith 2005
-
- Smith DK, Grohskopf LA, Black RJ. US Department of Health and Human Services. Antiretroviral postexposure prophylaxis after sexual, injection‐drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the U.S. Department of Health and Human Services. . MMWR Recomm Rep 2005;54(RR‐2):1‐20.. - PubMed
Subbarao 2006
-
- Subbarao S, Otten RA, Ramos A, Kim C, Jackson E, Monsour M, et al. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. Journal of Infectious Diseases October 2006;194(7):874‐6. - PubMed
WHO 2011
-
- WHO. GLOBAL HIV/AIDS RESPONSE, Epidemic update and health sector progress towards Universal Access. Progress Report 2011.
Youle 2003
-
- Youle M, Wainberg M. Pre‐exposure chemoprophylaxis (PREP) as an HIV prevention strategy. Journal of the International Association of Physicians in AIDS Care 2003;2(3):102‐5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

