Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents
- PMID: 22786509
- PMCID: PMC6599878
- DOI: 10.1002/14651858.CD007986.pub2
Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents
Update in
-
Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents.Cochrane Database Syst Rev. 2023 Apr 14;4(4):CD007986. doi: 10.1002/14651858.CD007986.pub3. Cochrane Database Syst Rev. 2023. PMID: 37058600 Free PMC article.
Abstract
Background: Attention deficit hyperactivity disorder (ADHD) is a major problem in children and adolescents, characterised by age-inappropriate levels of inattention, hyperactivity and impulsivity, and is associated with long-term social, academic and mental health problems. The stimulant medications methylphenidate and amphetamine are the most frequently used treatments for ADHD, but these are not always effective and can be associated with side effects. Clinical and biochemical evidence suggests that deficiencies of polyunsaturated fatty acids (PUFA) could be related to ADHD. Children and adolescents with ADHD have been shown to have significantly lower plasma and blood concentrations of PUFA and, in particular, lower levels of omega-3 PUFA. These findings suggest that PUFA supplementation may reduce the attention and behaviour problems associated with ADHD.
Objectives: To compare the efficacy of PUFA to other forms of treatment or placebo in treating the symptoms of ADHD in children and adolescents.
Search methods: We searched the following databases in August 2011: CENTRAL (The Cochrane Library 2011, Issue 2), MEDLINE (1948 to July Week 3, 2011), EMBASE (1980 to 2011 Week 29), PsycINFO (1806 to current), CINAHL (1937 to current), BIOSIS (1969 to 30 July 2011), Science Citation Index (1970 to 30 July 2011), Social Science Citation Index (1970 to 30 July 2011), Conference Proceedings Citation Index - Science (1990 to 30 July 2011), Conference Proceedings Citation Index - Social Science and Humanities (1990 to 30 July 2011), Cochrane Database of Systematic Reviews (2011, Issue 7), DARE (2011 Issue 2), Dissertation Abstracts (via Dissertation Express) and the metaRegister of Controlled Trials (mRCT). In addition, we searched the following repositories for theses on 2 August 2011: DART, NTLTD and TROVE. We also checked reference lists of relevant studies and reviews for additional references.
Selection criteria: Two review authors independently assessed the results of the database searches. We resolved any disagreements regarding the selection of studies through consensus or, if necessary, by consultation with a third member of the review team.
Data collection and analysis: Two members of the review team independently extracted details of participants and setting, interventions, methodology and outcome data. If differences were identified, we resolved them by consensus or referral to a third member of the team. We made all reasonable attempts to contact the authors where further clarification or missing data were needed.
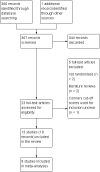
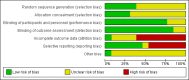
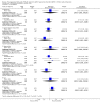
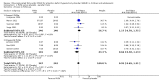
Main results: We included 13 trials with 1011 participants in the review. After screening 366 references, we considered 23 relevant and obtained the full text for consideration. We excluded five papers and included 18 papers describing the 13 trials. Eight of the included trials had a parallel design: five compared an omega-3 PUFA supplement to placebo; two compared a combined omega-3 and omega-6 supplement to placebo, and one compared an omega-3 PUFA to a dietary supplement. Five of the included trials had a cross-over design: two compared combined omega-3/6 PUFA to placebo; two compared omega-6 PUFA with placebo; one compared omega-3 to omega-6 PUFA, and one compared omega-6 PUFA to dexamphetamine. Supplements were given for a period of between four and 16 weeks.There was a significantly higher likelihood of improvement in the group receiving omega-3/6 PUFA compared to placebo (two trials, 97 participants; risk ratio (RR) 2.19, 95% confidence interval (CI) 1.04 to 4.62). However, there were no statistically significant differences in parent-rated ADHD symptoms (five trials, 413 participants; standardised mean difference (SMD) -0.17, 95% CI -0.38 to 0.03); inattention (six trials, 469 participants; SMD -0.04, 95% CI -0.29 to 0.21) or hyperactivity/impulsivity (five trials, 416 participants; SMD -0.04, 95% CI -0.25 to 0.16) when all participants receiving PUFA supplements were compared to those receiving placebo.There were no statistically significant differences in teacher ratings of overall ADHD symptoms (four trials, 324 participants; SMD 0.05, 95% CI -0.18 to 0.27); inattention (three trials, 260 participants; SMD 0.26, 95% CI -0.22 to 0.74) or hyperactivity/impulsivity (three trials, 259 participants; SMD 0.10, 95% CI -0.16 to 0.35).There were also no differences between groups in behaviour, side effects or loss to follow-up.Overall, there were no other differences between groups for any other comparison.
Authors' conclusions: Overall, there is little evidence that PUFA supplementation provides any benefit for the symptoms of ADHD in children and adolescents. The majority of data showed no benefit of PUFA supplementation, although there were some limited data that did show an improvement with combined omega-3 and omega-6 supplementation.It is important that future research addresses current weaknesses in this area, which include small sample sizes, variability of selection criteria, variability of the type and dosage of supplementation, short follow-up times and other methodological weaknesses.
Conflict of interest statement
Donna Gillies: none known. Sagar Lad: has been an investigator on a pre‐post trial of PUFA for symptoms of ADHD (Joshi 2006). Matthew Leach: none known. Melissa Ross: none known. John Sinn: none known.
Figures







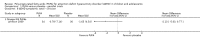
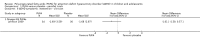

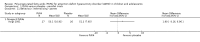
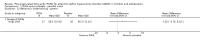

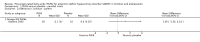



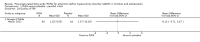
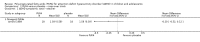
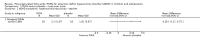
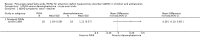




Comment in
-
Review: little evidence that PUFA supplementation improves symptoms in ADHD.Evid Based Ment Health. 2013 Feb;16(1):12. doi: 10.1136/eb-2012-100986. Epub 2012 Oct 23. Evid Based Ment Health. 2013. PMID: 23093692 No abstract available.
References
References to studies included in this review
-
- Aman MG, Mitchell EA, Turbott SH. The effects of essential fatty acid supplementation by Efamol in hyperactive children. Journal of Abnormal Child Psychology 1987;15(1):75‐90. - PubMed
-
- Arnold LE, Kleykamp D, Votolato N, Gibson RA, Horrocks L. Potential link between dietary intake of fatty acids and behavior: pilot exploration of serum lipids in attention‐deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 1994;4(3):171‐82.
- Arnold LE, Kleykamp D, Votolato NA, Taylor WA, Kontras SB, Tobin K. Gamma‐linolenic acid for attention‐deficit hyperactivity disorder: placebo‐controlled comparison to D‐amphetamine. Biological Psychiatry 1989;25(2):222‐8. - PubMed
- Arnold LE, Pinkham SM, Votolato N. Does zinc moderate essential fatty acid and amphetamine treatment of attention‐deficit/hyperactivity disorder?. Journal of Child and Adolescent Psychopharmacology 2000;10(2):111‐7. - PubMed
-
- Brue AW, Oakland TD, Evans RA. The use of a dietary supplement combination and an essential fatty acid as an alternative and complementary treatment for children with attention‐deficit/hyperactivity disorder. The Sciientific Review of Alternative Medicine 2001;5(4):187‐94.
-
- Gustafsson PA, Birberg‐Thornberg U, Duchén K, Landgren M, Malmberg K, Pelling H, et al. EPA supplementation improves teacher‐rated behaviour and oppositional symptoms in children with ADHD. Acta Pædiatrica 2010;99(10):1540‐9. - PubMed
References to studies excluded from this review
-
- Anon. Omega‐3/omega‐6 fatty acids and ADHD. Brown University Child & Adolescent Psychopharmacology Update 2009;11(4):4‐5.
-
- Doebel JK, Keller G, Zierau M‐T. Dietary therapy for children with ADHD. Deutsche Apotheker Zeitung 2005;145(24):54‐7.
-
- Harding KL, Judah RD, Gant C. Outcome‐based comparison of Ritalin versus food‐supplement treated children with AD/HD. Alternative Medicine Review 2003;8(3):319‐30. - PubMed
-
- Joshi K, Lad S, Kale M, Patwardhan B, Mahadik SP, Patni B, et al. Supplementation with flax oil and vitamin C improves the outcome of attention deficit hyperactivity disorder (ADHD). Prostaglandins Leukotrienes and Essential Fatty Acids 2006;74(1):17‐21. - PubMed
-
- Richardson AJ, Puri BK. A randomized double‐blind, placebo‐controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD‐related symptoms in children with specific learning difficulties. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2002;26(2):233‐9. - PubMed
References to ongoing studies
-
- Bos D, Belle J, Weusten J, Hoeksma M, Wiseman S, Durston S. Effects of N‐3 fatty acids (DHA and EPA) on cognitive control and associated brain activity in ADHD: a double‐blind placebo controlled study. European Child and Adolescent Psychiatry2010; Vol. 19, issue Suppl 1:S69.
-
- Omega‐3 supplementation and attention‐deficit‐hyperactivity disorder (ADHD). Ongoing studyApril 2009.
-
- PAD‐Study ‐ Efficacy of polyunsaturated fatty acids in children and adolescents with attention deficit/hyperactivity disorder (ADHD). Ongoing study Recruiting 1 August 2011.
-
- Clinical trial to evaluate the efficacy and the tolerance of an omega 3 fatty acids supplementation in ADHD children. Ongoing studyOctober 2008.
-
- Randomised controlled trial investigating effect of supplementation with omega‐3 fatty acids EPA and DHA versus omega‐6 fatty acid LA on ADHD symptoms and learning difficulties in 7‐12 year old children. Ongoing study 16 July 2007.
Additional references
-
- Achenbach TM, Edelbrock CS. Manual for the Child Behavior Checklist. Burlington, VT: University of Vermont, Department of Psychiatry, 1983.
-
- Agostoni C. Role of long‐chain polyunsaturated fatty acids in the first year of life. Journal of Pediatric Gastroenterology and Nutrition 2008;47 (Suppl 2):S41‐4. - PubMed
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington DC: American Psychiatric Association, 1994.
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (text revision). Washington, DC: American Psychiatric Association, 2000.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

