Vascular-endothelial-growth-factor (VEGF) targeting therapies for endocrine refractory or resistant metastatic breast cancer
- PMID: 22786517
- PMCID: PMC12066189
- DOI: 10.1002/14651858.CD008941.pub2
Vascular-endothelial-growth-factor (VEGF) targeting therapies for endocrine refractory or resistant metastatic breast cancer
Abstract
Background: Vascular-endothelial-growth-factor (VEGF) is a key mediator of angiogenesis. VEGF-targeting therapies have shown significant benefits and been successfully integrated in routine clinical practice for other types of cancer, such as metastatic colorectal cancer. By contrast, individual trial results in metastatic breast cancer (MBC) are highly variable and their value is controversial.
Objectives: To evaluate the benefits (in progression-free survival (PFS) and overall survival (OS)) and harms (toxicity) of VEGF-targeting therapies in patients with hormone-refractory or hormone-receptor negative metastatic breast cancer.
Search methods: Searches of CENTRAL, MEDLINE, EMBASE, the Cochrane Breast Cancer Group's Specialised Register, registers of ongoing trials and proceedings of conferences were conducted in January and September 2011, starting in 2000. Reference lists were scanned and members of the Cochrane Breast Cancer Group, experts and manufacturers of relevant drug were contacted to obtain further information. No language restrictions were applied.
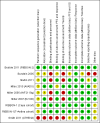
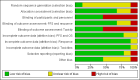
Selection criteria: Randomised controlled trials (RCTs) to evaluate treatment benefit and non-randomised studies in the routine oncology practice setting to evaluate treatment harms.
Data collection and analysis: We performed data collection and analysis according to the published protocol. Individual patient data was sought but not provided. Therefore, the meta-analysis had to be based on published data. Summary statistics for the primary endpoint (PFS) were hazard ratios (HRs).
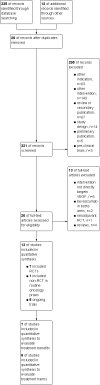
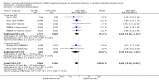
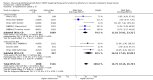
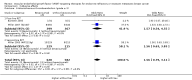
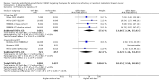
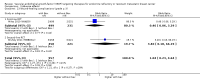
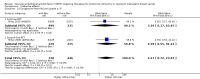
Main results: We identified seven RCTs, one register, and five ongoing trials from a total of 347 references. The published trials for VEGF-targeting drugs in MBC were limited to bevacizumab. Four trials, including a total of 2886 patients, were available for the comparison of first-line chemotherapy, with versus without bevacizumab. PFS (HR 0.67; 95% confidence interval (CI) 0.61 to 0.73) and response rate were significantly better for patients treated with bevacizumab, with moderate heterogeneity regarding the magnitude of the effect on PFS. For second-line chemotherapy, a smaller, but still significant benefit in terms of PFS could be demonstrated for patients treated with bevacizumab (HR 0.85; 95% CI 0.73 to 0.98), as well as a benefit in tumour response. However, OS did not differ significantly, neither in first- (HR 0.93; 95% CI 0.84 to 1.04), nor second-line therapy (HR 0.98; 95% CI 0.83 to 1.16). Quality of life (QoL) was evaluated in four trials but results were published for only two of these with no relevant impact. Subgroup analysis stated a significant greater benefit for patients with previous (taxane) chemotherapy and patients with hormone-receptor negative status. Regarding toxicity, data from RCTs and registry data were consistent and in line with the known toxicity profile of bevacizumab. While significantly higher rates of adverse events (AEs) grade III/IV (odds ratio (OR) 1.77; 95% CI 1.44 to 2.18) and serious adverse events (SAEs) (OR 1.41; 95% CI 1.13 to 1.75) were observed in patients treated with bevacizumab, rates of treatment-related deaths were lower in patients treated with bevacizumab (OR 0.60; 95% CI 0.36 to 0.99).
Authors' conclusions: The overall patient benefit from adding bevacizumab to first- and second-line chemotherapy in metastatic breast cancer can at best be considered as modest. It is dependent on the type of chemotherapy used and limited to a prolongation of PFS and response rates in both first- and second-line therapy, both surrogate parameters. In contrast, bevacizumab has no significant impact on the patient-related secondary outcomes of OS or QoL, which indicate a direct patient benefit. For this reason, the clinical value of bevacizumab for metastatic breast cancer remains controversial.
Conflict of interest statement
Professor Christoph Thomssen has received speaker honoraria, participated on the Advisory Board and received research funding from Roche.
Figures



















Update of
- doi: 10.1002/14651858.CD008941
References
References to studies included in this review
Brufsky 2011 (RIBBON‐2) {published and unpublished data}
-
- Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O'Neill V, et al. RIBBON‐2: A randomized, double‐blind, placebo‐controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second‐line treatment of human epidermal growth factor receptor 2‐negative metastatic breast cancer. Journal of Clinical Oncology 2011;29(32):4286‐93. [DOI: 10.1200/JCO.2010.34.1255] - DOI - PubMed
Burstein 2005 {published and unpublished data}
-
- Burstein HJ, Spigel D, Kindsvogel K, Parker LM, Bunnell CA, Partridge AH, et al. Metronomic chemotherapy with and without bevacizumab for advanced breast cancer: a randomized phase II study. Breast Cancer Research and Treatment 2005;94(Supplementary 1):S6.
Martin 2011 {published data only}
-
- Martin M, Roche H, Pinter T, Crown J, Kennedy MJ, Provencher L, et al. Motesanib, or open‐label bevacizumab, in combination with paclitaxel, as first‐line treatment for HER2‐negative locally recurrent or metastatic breast cancer: a phase 2, randomised, double‐blind, placebo‐controlled study. Lancet Oncology 2011; Vol. 12, issue 4:369‐76. - PubMed
Miles 2010 (AVADO) {published data only}
-
- Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, et al. Bevacizumab plus docetaxel compared with placebo plus docetaxel for the first‐line treatment of HER‐2‐negative, metastatic breast cancer. Journal of Clinical Oncology 2010;28(20):3239‐47. - PubMed
Miller 2005 (AVF2119g) {published data only}
-
- Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. Journal of Clinical Oncology 2005;23(4):792‐9. - PubMed
Miller 2007 (E2100) {published data only}
-
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel. New England Journal of Medicine 2007;357:2666‐76. - PubMed
RIBBON‐1 (Cape cohort) {published and unpublished data}
-
- Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, et al. RIBBON‐1: randomized, double‐blind, placebo‐controlled, phase III trial of chemotherapy with or without bevacizumab (B) for first‐line treatment of Human Epidermal Growth Factor Receptor 2‐negative, locally recurrent or metastatic breast cancer. Journal of Clinical Oncology 2011;29(10):1252‐60. - PubMed
RIBBON‐1(T+Anthra cohort) {published and unpublished data}
-
- Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, et al. RIBBON‐1: randomized, double‐blind, placebo‐controlled, phase III trial of chemotherapy with or without bevacizumab (B) for first‐line treatment of HER2‐negative locally recurrent or metastatic breast cancer (MBC). Journal of Clinical Oncology 2011;29(10):1252‐60. - PubMed
Smith 2011 (ATHENA) {published data only}
-
- Smith IE, Pierga JY, Biganzoli L, Cortés‐Funes H, Thomssen C, Pivot X, et al. First‐line bevacizumab plus taxane‐based chemotherapy for locally recurrent or metastatic breast cancer: safety and efficacy in an open‐label. Annals of Oncology 2011;22(3):595‐602. - PubMed
References to studies excluded from this review
Baar 2009 {published data only}
Baidas 2000 {published data only}
-
- Baidas SM, Winer EP, Fleming GF, Harris L, Pluda JM, Crawford JG, et al. Phase II evaluation of thalidomide in patients with metastatic breast cancer. Journal of Clinical Oncology 2000;18(14):2710‐7. - PubMed
Barrios 2010 {published data only}
Cameron 2010 {published data only}
Mackey 2009 {published data only}
-
- Mackey J, Gelmon K, Martin M, McCarthy N, Pinter T, Rupin M, et al. TRIO‐012: A multicenter, multinational, randomized, double‐blind phase III study of imc‐1121b plus docetaxel versus placebo plus docetaxel in previously untreated patients with her2‐negative, unresectable, locally recurrent or metastatic breast cancer. Clinical Breast Cancer 2009;9(4):258‐61. - PubMed
Mayer 2010 {published data only}
-
- Mayer EL, Dhakil S, Patel T, Sundaram S, Fabian C, Kozloff M, et al. SABRE‐B: an evaluation of paclitaxel and bevacizumab with or without sunitinib as first‐line treatment of metastatic breast cancer. Annals of Oncology 2010;21(12):2370‐6. - PubMed
NCT01303679 {published data only}
-
- Micheau D. First line treatment of bevacizumab‐taxane vs bevacizumab‐exemestane in metastatic breast cancer. http://clinicaltrials.gov/ct2/show/NCT01303679 (assessed on 4 October 2011).
Rugo 2009 {published data only}
-
- Rugo HS, Campone M, Amadori D, Wardley A, Villa E, Conte PF, et al. Randomized phase II study of weekly versus every‐3‐week ixabepilone plus bevacizumab (ixa/bev) versus paclitaxel plus bev (pac/bev) as first‐line therapy for metastatic breast cancer (MBC). Journal of Clinical Oncology 2009;27(15):1029.
Wildiers 2010 {published data only}
-
- Wildiers H, Fontaine C, Vuylsteke P, Martens M, Canon JL, Wynendaele W, et al. Multicenter phase II randomized trial evaluating antiangiogenic therapy with sunitinib as consolidation after objective response to taxane chemotherapy in women with HER2‐negative metastatic breast cancer. Breast Cancer Research and Treatment 2010;123(2):463‐9. - PubMed
References to ongoing studies
Guirado‐Risueño 2010 {published data only}
-
- Guirado‐Risueño M, Perez Manga G, Rifa J, Perez Carrion RM, García López MJ, Velasco A, et al. Multicentric, observational, transversal study to describe the clinical profile of patients with metastatic breast cancer (MBC) treated with first‐line bevacizumab (TRANSBREAST): preliminary results. Journal of Clinical Oncology. 2010; Vol. 28:15 supplementary, abstract 1143.
Hegewisch‐Becker 2011 {published data only}
-
- Hegewisch‐Becker S, Lerchenmuller CA, Welt A, Decker T, Just M, Steffens C, et al. Capecitabine (Cap) combined with bevacizumab (Bev) with or without vinorelbine (Vin) in first‐line metastatic breast cancer (MBC): First safety results from the randomized CARIN trial. Journal of Clinical Oncology 2011;29(Supplementary):Abstract 1044.
Klare 2011 {published data only}
-
- Klare P, Foerster FG, Geberth M, Schneeweiss A, Tesch H, Kuemmel S, et al. Efficacy and safety of first‐line bevacizumab (Bev) combined with paclitaxel (Pac): An observational study in 786 patients (pts) with HER2‐negative metastatic breast cancer (mBC). Journal of Clinical Oncology 2011;29(Supplementary):Abstract 1079.
NCT00217672 {published data only}
-
- Docetaxel with bevacizumab as first‐line therapy in treating women with stage IV breast cancer. http://www.clinicaltrials.gov/ct2/results?term=NCT00217672 (assessed on 15 June 2011).
NCT00856492 {published data only}
-
- Paclitaxel albumin‐stabilized nanoparticle formulation, doxorubicin, cyclophosphamide, and pegfilgrastim with or without bevacizumab in treating women with inflammatory or locally advanced breast cancer. http://www.clinicaltrials.gov/ct2/results?term=NCT00856492 (assessed 15 June 2011).
Additional references
Bear 2011
-
- Bear H D, Tang G, Rastogi P, Geyer CE, Robidoux A, Atkins JN. The effect on pCR of bevacizumab and/or antimetabolites added to standard neoadjuvant chemotherapy: NSABP protocol B‐40. Journal of Clinical Oncology. 2011; Vol. 29 (supplementary; abstract LBA1005).
Bergers 2008
Boneberg 2009
Borenstein 2008
-
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta‐analysis. Chichester (UK): John Wiley & Sons, 2008.
Brady 1997
-
- Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy‐Breast quality‐of‐life instrument. Journal of Clinical Oncology 1997;15:974‐86. - PubMed
Broglio 2009
Brown 1995
-
- Brown LF, Berse B, Jackman RW, Tognazzi K, Guidi AJ, Dvorak HF, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Human Pathology 1995;26(1):86‐91. - PubMed
Burzykowski 2008
-
- Burzykowski T, Buyse M, Piccart‐Gebhart MJ, Sledge G, Carmichael J, Lück HJ, et al. Evaluation of tumor response, disease control, progression‐free survival, and time to progression as potential surrogate end points in metastatic breast cancer. Journal of Clinical Oncology 2008;26(12):1987‐92. - PubMed
Carey 2011
-
- Carey LA. Directed therapy of subtypes of triple‐negative breast cancer. The Oncologist 2011;16(Supplementary 1):71‐8. - PubMed
Casanovas 2005
-
- Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late‐stage pancreatic islet tumors. Cancer Cell 2005;8(4):299‐309. - PubMed
Cella 2011
-
- Cella D, Wang M, Wagner L, Miller K. Survival‐adjusted health‐related quality of life (HRQL) among patients with metastatic breast cancer receiving paclitaxel plus bevacizumab versus paclitaxel alone: results from Eastern Cooperative Oncology Group Study 2100 (E2100). Breast Cancer Research and Treatment 2011;130(3):855‐61. [PUBMED: PMID: 21874312 ] - PMC - PubMed
Choueiri 2011
-
- Choueiri TK, Mayer EL, Je Y, Rosenberg JE, Nguyen PL, Azzi GR, et al. Congestive heart failure risk in patients with breast cancer treated with bevacizumab. Journal of Clinical Oncology 2011;29(6):632‐8. - PubMed
Cortes 2011
-
- Cortes J, Calvo V, Ramírez‐Merino N, O'Shaughnessy J, Brufsky A, Robert N, et al. Adverse events risk associated with bevacizumab addition to breast cancer chemotherapy: a meta‐analysis. Annals of Oncology 2012; Vol. 23, issue 5:1130‐7. - PubMed
Cox 1972
-
- Cox DR. Regression models and life tables. Journal of the Royal Statistical Society 1972;34(Series B):187‐220.
Cuppone 2011
-
- Cuppone F, Bria E, Vaccaro V, Puglisi F, Fabi A, Sperduti I, et al. Magnitude of risks and benefits of the addition of bevacizumab to chemotherapy for advanced breast cancer patients: Meta‐regression analysis of randomized trials. Journal of Experimental & Clinical Cancer Research 2011;30:54. - PMC - PubMed
Deeks 2003
-
- Deeks JJ, Dines J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non‐randomized intervention studies. Health Technology Assessment 2003;7(27):iii‐x, 1‐173. - PubMed
Ebos 2011
Ellis 2008
-
- Ellis LM, Hicklin DJ. VEGF‐targeted therapy: mechanisms of anti‐tumour activity. Nature Reviews Cancer 2008;8(8):579‐91. - PubMed
Elston 1991
-
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. 1. The value of histological grade in breast cancer: experience from a large study with long term follow‐up. Histopathology 1991;19:403‐10. - PubMed
Ferrara 2004
-
- Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti‐VEGF antibody for treating cancer. Nature Reviews. Drug Discovery 2004;3(5):391‐400. - PubMed
Garcia 2007
-
- Garcia M, Jemal A, Ward EM, Center MM, Hao Y, Thun MJ. Global cancer facts and figures 2007. www.cancer.org/downloads/STT/Global_Facts_and_Figures_2007_rev2.pdf (accessed 06 April 2010):10.
Gasparini 1997
-
- Gasparini G, Toi M, Gion M, Verderio P, Dittadi R, Hanatani M, et al. Prognostic significance of vascular endothelial growth factor protein in node‐negative breast carcinoma. Journal of the National Cancer Institute 1997;89(2):139‐47. - PubMed
Gerber 2011
-
- Gerber B, Eidtmann H, Rezai M, Fasching P, Tesc H, Eggemann H, et al. Neoadjuvant bevacizumab and anthracycline/taxane‐based chemotherapy in 686 triple‐negative primary breast cancers: Secondary endpoint analysis of the GeparQuinto study (GBG 44). Journal of Clinical Oncology. 2011; Vol. 29 (supplementary; abstract 1006).
Gray 2009
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in medicine 2002;21:1539‐58. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org: The Cochrane Collaboration, 2011.
Hu 2009
Hudis 2011
-
- Hudis CA, Gianni L. Triple‐negative breast cancer: an unmet medical need. The Oncologist 2011;16(Supplementary 1):1‐11. - PubMed
Hurwitz 2004
-
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinoteca, fluorouracil, and leucovorin for metastatic colorectal cancer. New England Journal of Medicine 2004;350(23):2335‐42. - PubMed
Lee 2011
-
- Lee JB, Woo OH, Park KH, Woo SU, Yang DS, Kim AR, et al. Bevacizumab for salvage treatment of metastatic breast cancer: a systemic review and meta‐analysis of randomized controlled trials. Investigational New Drugs 2011;29:182‐8. - PubMed
Linderholm 2009
-
- Linderholm BK, Hellborg H, Johansson U, Elmberger G, Skoog L, Lehtiö J, et al. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple‐negative breast cancer. Annals of Oncology 2009;20(10):1639‐46. - PubMed
Loke 2008
-
- Loke YK, Price D, Herxheimer A. Chapter 14: Adverse effects. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:433‐48.
Marty 2008
-
- Marty M, Pivot X. The potential of anti‐vascular endothelial growth factor therapy in metastatic breast cancer: Clinical experience with anti‐angiogenic agents, focusing on bevacizumab. European Journal of Cancer 2008;44:912‐20. - PubMed
Miller 2007
-
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. New England Journal of Medicine 2007;357:2666‐76. - PubMed
NCI 1999
-
- National Cancer Institute. Common Toxicity Criteria (CTC Version 2.0). http://www.eortc.be/services/doc/ctc/ctcv20_4‐30‐992.pdf. - PubMed
NCI 2006
-
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/... (15/08/2011) 2006.
Ng 2008
-
- Ng HKT, Filardo G, Zheng G. Confidence interval estimating procedures for standardized incidence rates. Computational Statistics & Data Analysis 2008;52(7):3501‐16.
O'Shaughnessy 2010
-
- O'Shaughnessy J, Miles D, Gray RJ, Dieras V, Perez EA, Zon R, et al. A meta‐analysis of survival data from three randomized trials of bevacizumab (BV) and first‐line chemotherapy as treatment for patients with metastatic breast cancer (MBC). Journal of Clinical Oncology 2010;15s:Abstract 1005.
Papanikolaou 2006
Parmar 1998
-
- Parmar MKB, Torri V, Steward L. Extracting summary statistics to perform meta‐analysis of the published literature for survival endpoints. Statistics in Medicine 1998;17:2814‐34. - PubMed
Pivot 2011
-
- Pivot X, Schneeweiss A, Verma S, Thomssen C, Passos‐Coelho JL, Benedetti G, et al. Efficacy and safety of bevacizumab in combination with docetaxel for the first‐line treatment of elderly patients with locally recurrent or metastatic breast cancer: Results from AVADO. European Journal of Cancer 2011;47(16):2387‐95. - PubMed
Pàez‐Ribes 2009
Ranpura 2011
-
- Ranpura V, Hapani S, Wu S. Treatment‐related mortality with bevacizumab in cancer patients: a meta‐analysis. JAMA 2011;305(5):487–94. - PubMed
RevMan 5 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Rugo 2011
-
- Rugo H. Occams Razor or Hickams Dictum: The Law of Parsimony Wins? or Does it?. ASCO Annual Meeting Discussion Oral Abstract Session 2011.
Saad 2010
-
- Saad E, Katz A, Buyse MM. Overall survival and post progression survival in advanced breast cancer: a review of recent randomized clinical trials. Journal of Clinical Oncology 2010; Vol. 28, issue 11:1958‐62. - PubMed
Scappaticci 2007
-
- Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. Journal of the National Cancer Institute 2007;99(16):1232‐9. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Shea 2009
-
- Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. Journal of Clinical Epidemiology 2009;62(10):1013‐20. - PubMed
Therasse 2000
-
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 2000;92(3):205‐16. - PubMed
Thomssen 2009
-
- Thomssen C, Pierga J, Pritchard K, Biganzoli L, Cortes‐Funes H, Petrakova K, et al. First‐line bevacizumab combination therapy in triple‐ negative locally recurrent/metastatic breast cancer: subpopulation analysis of study MO19391 in >2000 patients. San Antonio Breast Cancer Symposium. San Antonio, Texas, December 13, 2009; Vol. abstract 6093.
Tierney 2007
-
- Tierney JF, Stewart L, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007; Vol. 8, issue http://www.trialsjournal.com/content/8/1/16. - PMC - PubMed
Valachis 2010
-
- Valachis A, Polyzos NP, Patsopoulos NA, Georgoulias V, Mavroudis D, Mauri D. Bevacizumab in metastatic breast cancer: a meta‐analysis of randomized controlled trials. Breast Cancer Research and Treatment 2010;122(1):1‐7. - PubMed
Van Houwelingen 2002
-
- Houwelingen HC, Arends LR, Stijnen T. Tutorial in biostatistics: advanced methods in meta‐analysis: multivariate approach and meta‐regression. Statistics in medicine 2002;21:589‐624. - PubMed
Vandenbroucke 2004
Vandenbroucke 2006
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

