Hepatitis A immunisation in persons not previously exposed to hepatitis A
- PMID: 22786522
- PMCID: PMC6823267
- DOI: 10.1002/14651858.CD009051.pub2
Hepatitis A immunisation in persons not previously exposed to hepatitis A
Update in
-
Hepatitis A immunisation in persons not previously exposed to hepatitis A.Cochrane Database Syst Rev. 2019 Dec 17;12(12):CD009051. doi: 10.1002/14651858.CD009051.pub3. Cochrane Database Syst Rev. 2019. PMID: 31846062 Free PMC article. Review.
Abstract
Background: In many parts of the world, hepatitis A infection represents a significant cause of morbidity and socio-economic loss. Whilst hepatitis A vaccines have the potential to prevent disease, the degree of protection afforded against clinical outcomes and within different populations remains uncertain. There are two types of hepatitis A virus (HAV) vaccine, inactivated and live attenuated. It is important to determine the efficacy and safety for both vaccine types.
Objectives: To determine the clinical protective efficacy, sero-protective efficacy, and safety and harms of hepatitis A vaccination in persons not previously exposed to hepatitis A.
Search methods: We searched The Cochrane Hepato-Biliary Group Controlled Trials Register, The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded, and China National Knowledge Infrastructure (CNKI) up to November 2011.
Selection criteria: Randomised clinical trials comparing HAV vaccine with placebo, no intervention, or appropriate control vaccines in participants of all ages.
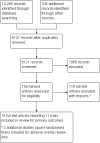
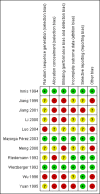
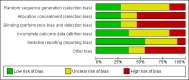
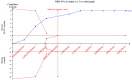
Data collection and analysis: Data extraction and risk of bias assessment were undertaken by two authors and verified by a third author. Where required, authors contacted investigators to obtain missing data. The primary outcome was the occurrence of clinically apparent hepatitis A (infectious hepatitis). The secondary outcomes were lack of sero-protective anti-HAV immunoglobulin G (IgG), and number and types of adverse events. Results were presented as relative risks (RR) with 95% confidence intervals (CI). Dichotomous outcomes were reported as risk ratio (RR) with 95% confidence interval (CI), using intention-to-treat analysis. We conducted assessment of risk of bias to evaluate the risk of systematic errors (bias) and trial sequential analyses to estimate the risk of random errors (the play of chance).
Main results: We included a total of 11 clinical studies, of which only three were considered to have low risk of bias; two were quasi-randomised studies in which we only addressed harms. Nine randomised trials with 732,380 participants addressed the primary outcome of clinically confirmed hepatitis A. Of these, four trials assessed the inactivated hepatitis A vaccine (41,690 participants) and five trials assessed the live attenuated hepatitis A vaccine (690,690 participants). In the three randomised trials with low risk of bias (all assessing inactivated vaccine), clinically apparent hepatitis A occurred in 9/20,684 (0.04%) versus 92/20,746 (0.44%) participants in the HAV vaccine and control groups respectively (RR 0.09, 95% CI 0.03 to 0.30). In all nine randomised trials, clinically apparent hepatitis A occurred in 31/375,726 (0.01%) versus 505/356,654 (0.18%) participants in the HAV vaccine and control groups respectively (RR 0.09, 95% CI 0.05 to 0.17). These results were supported by trial sequential analyses. Subgroup analyses confirmed the clinical effectiveness of both inactivated hepatitis A vaccines (RR 0.09, 95% CI 0.03 to 0.30) and live attenuated hepatitis A vaccines (RR 0.07, 95% CI 0.03 to 0.17) on clinically confirmed hepatitis A. Inactivated hepatitis A vaccines had a significant effect on reducing the lack of sero-protection (less than 20 mIU/L) (RR 0.01, 95% CI 0.00 to 0.03). No trial reported on a sero-protective threshold less than 10 mIU/L. The risk of both non-serious local and systemic adverse events was comparable to placebo for the inactivated HAV vaccines. There were insufficient data to draw conclusions on adverse events for the live attenuated HAV vaccine.
Authors' conclusions: Hepatitis A vaccines are effective for pre-exposure prophylaxis of hepatitis A in susceptible individuals. This review demonstrated significant protection for at least two years with the inactivated HAV vaccine and at least five years with the live attenuated HAV vaccine. There was evidence to support the safety of the inactivated hepatitis A vaccine. More high quality evidence is required to determine the safety of live attenuated vaccines.
Conflict of interest statement
None known
Figures




















Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Vaccines for preventing influenza in healthy children.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD004879. doi: 10.1002/14651858.CD004879.pub5. Cochrane Database Syst Rev. 2018. PMID: 29388195 Free PMC article.
-
Direct-acting antivirals for chronic hepatitis C.Cochrane Database Syst Rev. 2017 Sep 18;9(9):CD012143. doi: 10.1002/14651858.CD012143.pub3. Cochrane Database Syst Rev. 2017. PMID: 28922704 Free PMC article.
-
Vitamin D supplementation for chronic liver diseases in adults.Cochrane Database Syst Rev. 2017 Nov 3;11(11):CD011564. doi: 10.1002/14651858.CD011564.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2021 Aug 25;8:CD011564. doi: 10.1002/14651858.CD011564.pub3. PMID: 29099543 Free PMC article. Updated.
Cited by
-
Cost-effective analysis of hepatitis A vaccination in Kerala state, India.PLoS One. 2024 Jun 27;19(6):e0306293. doi: 10.1371/journal.pone.0306293. eCollection 2024. PLoS One. 2024. PMID: 38935781 Free PMC article.
-
Hepatitis A: Epidemiology, Natural History, Unusual Clinical Manifestations, and Prevention.Gastroenterol Clin North Am. 2020 Jun;49(2):191-199. doi: 10.1016/j.gtc.2020.01.002. Epub 2020 Mar 29. Gastroenterol Clin North Am. 2020. PMID: 32389358 Free PMC article. Review.
-
Early Seroreversion After 2 Doses of Hepatitis A Vaccination in Human Immunodeficiency Virus-Positive Patients: Incidence and Associated Factors.Hepatology. 2019 Aug;70(2):465-475. doi: 10.1002/hep.30495. Epub 2019 Feb 14. Hepatology. 2019. PMID: 30614542 Free PMC article.
-
Hepatitis a Vaccine as Opportunity of Primary Prevention for Food Handlers: A Narrative Review.Vaccines (Basel). 2023 Jul 21;11(7):1271. doi: 10.3390/vaccines11071271. Vaccines (Basel). 2023. PMID: 37515087 Free PMC article. Review.
-
Prevention and management of viral hepatitis in inflammatory bowel disease: a clinical practice guideline by the Korean Association for the Study of Intestinal Diseases.Intest Res. 2020 Jan;18(1):18-33. doi: 10.5217/ir.2019.09155. Epub 2020 Jan 30. Intest Res. 2020. PMID: 32013312 Free PMC article. Review.
References
References to studies included in this review
-
- Innis B, Snitbhan R, Kunasol R, Laorakpongse P, Poopatanakool T, Kozik C, et al. Field efficacy trial of inactivated hepatitis A vaccine among children in Thailand (an extended abstract). Vaccine 1992;10:S159.
- Innis B, Snitbhan R, Kunasol R, Laorakpongse P, Poopatanakool T, Kozik C, et al. Protection against hepatitis A by an inactivated vaccine. Journal of the American Medical Association 1994;271(17):1328‐34. - PubMed
-
- Jiang S, Huang J, Chen J. The epidemiological efficacy assessment of attenuated live hepatitis A vaccine in Masses in Liuzhou. Chinese Journal of Epidemiology 1995;16(3):140‐2. - PubMed
-
- Jiang W, Niu X. Observation on the efficacy of attenuated live hepatitis A vaccine's vaccination contingency. Modern Preventative Medicine 2001;28(1):59‐61.
-
- Li Y, Wu H, Xu T, Yuan G, Zhang A, Zhou T. Observation of immunogenicity and epidemiological efficacy assessment of attenuated live hepatitis A vaccine. Chinese Journal of Public Health 2000;16(8):737‐8.
-
- Luo D, Li R, Gong J. Epidemiological efficacy of standardized live attenuated hepatitis A vaccine (LA‐1 strain). Chinese Journal of Vaccination and Immunization 2004;10(2):210‐2.
References to studies excluded from this review
-
- Abarca K, Ibanez I, Flores J, Vial PA, Safary A, Potin M, et al. Efficacy of hepatitis A vaccination in children aged 12 to 24 months. Archives of Medical Research 2001;32(35):468‐72. - PubMed
-
- Abarca K, Ibanez I, Flores J, Vial PA, Safary A, Potin M, et al. Vaccination against hepatitis A in children aged 12 to 24 months [corrected]. Archives of Medical Research 2001;32(3):468‐72. - PubMed
-
- Ambrosch F, Wiedermann G, André F, Delem A, Gregor H, Hofmann H. Clinical and immunological investigation of a new combined hepatitis A and hepatitis B vaccine. Journal of Medical Virolology 1994;44(4):452. - PubMed
-
- Andre F. Randomized, cross‐over, controlled comparison of two inactivated hepatitis A vaccines. Vaccine 2001;20(23‐24):292‐3. - PubMed
-
- Anonymous. Is the hepatitis A vaccine an alternative for post‐exposure prophylaxis?. Journal of Family Practice 2008;57(2):82. - PubMed
Additional references
-
- Adams G, Gulliford M, Ukoumunne O, Eldridge S, Chinn S, Campbell M. Patterns of intra‐cluster correlation from primary care research to inform study design and analysis. Journal of Clinical Epidemiology 2004;57(8):785‐94. - PubMed
-
- Bell B. Global epidemiology of hepatitis A: implications for control strategies. In: Margolis H, Alter M, Liang J editor(s). Viral Hepatitis and Liver disease. London: International Medical Press, 2002:359‐65.
-
- Bell B. [Hepatitis A] Prevention. In: Thomas H, Lemon S, Zuckerman A editor(s). Viral Hepatitis. 3rd Edition. Oxford: Backwell Publishing, 2005:126‐45.
-
- Berge J, Drennan D, Jacobs R, Jakins A, Meyerhoff A, Stubblefield W, et al. The cost of hepatitis A infections in American adolescents and adults in 1997. Hepatology 2000;31(2):469‐73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

