The transcription factors BEL1 and SPL are required for cytokinin and auxin signaling during ovule development in Arabidopsis
- PMID: 22786869
- PMCID: PMC3426121
- DOI: 10.1105/tpc.112.100164
The transcription factors BEL1 and SPL are required for cytokinin and auxin signaling during ovule development in Arabidopsis
Abstract
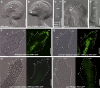
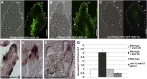
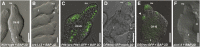
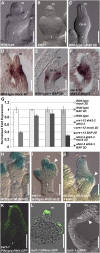
Hormones, such as auxin and cytokinin, are involved in the complex molecular network that regulates the coordinated development of plant organs. Genes controlling ovule patterning have been identified and studied in detail; however, the roles of auxin and cytokinin in ovule development are largely unknown. Here we show that key cytokinin pathway genes, such as isopentenyltransferase and cytokinin receptors, are expressed during ovule development. Also, in a cre1-12 ahk2-2 ahk3-3 triple mutant with severely reduced cytokinin perception, expression of the auxin efflux facilitator PIN-FORMED 1 (PIN1) was severely reduced. In sporocyteless/nozzle (spl/nzz) mutants, which show a similar phenotype to the cre1-12 ahk2-2 ahk3-3 triple mutant, PIN1 expression is also reduced. Treatment with the exogenous cytokinin N(6)-benzylaminopurine also altered both auxin distribution and patterning of the ovule; this process required the homeodomain transcription factor BELL1 (BEL1). Thus, this article shows that cytokinin regulates ovule development through the regulation of PIN1. Furthermore, the transcription factors BEL1 and SPL/NZZ, previously described as key regulators of ovule development, are needed for the auxin and cytokinin signaling pathways for the correct patterning of the ovule.
Figures


References
-
- Balasubramanian S., Schneitz K. (2000). NOZZLE regulates proximal-distal pattern formation, cell proliferation and early sporogenesis during ovule formation in Arabidopsis thaliana. Development 127: 4227–4238 - PubMed
-
- Balasubramanian S., Schneitz K. (2002). NOZZLE links proximal-distal and adaxial-abaxial pattern formation during ovule development in Arabidopsis thaliana. Development 129: 4291–4300 - PubMed
-
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 - PubMed
-
- Bennett M.J., Marchant A., Green H.G., May S.T., Ward S.P., Millner P.A., Walker A.R., Schulz B., Feldmann K.A. (1996). Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science 273: 948–950 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

