Radiofrequency ablation of lung tumors: imaging features of the postablation zone
- PMID: 22786987
- PMCID: PMC3393883
- DOI: 10.1148/rg.324105181
Radiofrequency ablation of lung tumors: imaging features of the postablation zone
Abstract
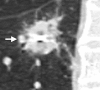
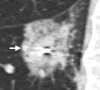
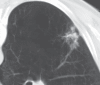
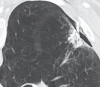
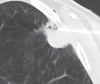
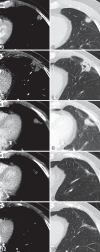
Radiofrequency ablation (RFA) is used to treat pulmonary malignancies. Although preliminary results are suggestive of a survival benefit, local progression rates are appreciable. Because a patient can undergo repeat treatment if recurrence is detected early, reliable post-RFA imaging follow-up is critical. The purpose of this article is to describe (a) an algorithm for post-RFA imaging surveillance; (b) the computed tomographic (CT) appearance, size, enhancement, and positron emission tomographic (PET) metabolic activity of the ablation zone; and (c) CT, PET, and dual-modality imaging with PET and CT (PET/CT) features suggestive of partial ablation or tumor recurrence and progression. CT is routinely used for post-RFA follow-up. PET and PET/CT have emerged as auxiliary follow-up techniques. CT with nodule densitometry may be used to supplement standard CT. Post-RFA follow-up was divided into three phases: early (immediately after to 1 week after RFA), intermediate (>1 week to 2 months), and late (>2 months). CT and PET imaging features suggestive of residual or recurrent disease include (a) increasing contrast material uptake in the ablation zone (>180 seconds on dynamic images), nodular enhancement measuring more than 10 mm, any central enhancement greater than 15 HU, and enhancement greater than baseline anytime after ablation; (b) growth of the RFA zone after 3 months (compared with baseline) and definitely after 6 months, peripheral nodular growth and change from ground-glass opacity to solid opacity, regional or distant lymph node enlargement, and new intrathoracic or extrathoracic disease; and (c) increased metabolic activity beyond 2 months, residual activity centrally or at the ablated tumor, and development of nodular activity.
Figures







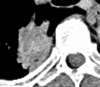
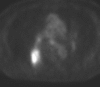


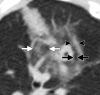



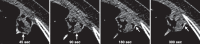
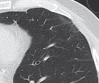

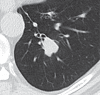
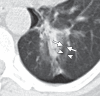
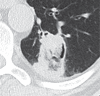
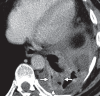

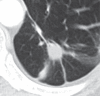



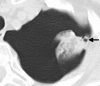

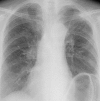











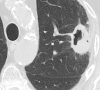



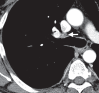





References
-
- American Cancer Society Cancer facts and figures 2011. American Cancer Society Web site. http://www.cancer.org/Research/CancerFactsFigures/Cancer FactsFigures/cancer-facts-figures-2011. Published 2011. Accessed October 18, 2011
-
- Davidson RS, Nwogu CE, Brentjens MJ, Anderson TM. The surgical management of pulmonary metastasis: current concepts. Surg Oncol 2001;10(1-2):35–42 - PubMed
-
- Mery CM, Pappas AN, Burt BM, et al. Diameter of non-small cell lung cancer correlates with long-term survival: implications for T stage. Chest 2005;128(5):3255–3260 - PubMed
-
- McGarry RC, Song G, des Rosiers P, Timmerman R. Observation-only management of early stage, medically inoperable lung cancer: poor outcome. Chest 2002;121(4):1155–1158 - PubMed
-
- Kyasa MJ, Jazieh AR. Characteristics and outcomes of patients with unresected early-stage non-small cell lung cancer. South Med J 2002;95(10):1149–1152 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

