New insights into ice growth and melting modifications by antifreeze proteins
- PMID: 22787007
- PMCID: PMC3481565
- DOI: 10.1098/rsif.2012.0388
New insights into ice growth and melting modifications by antifreeze proteins
Abstract
Antifreeze proteins (AFPs) evolved in many organisms, allowing them to survive in cold climates by controlling ice crystal growth. The specific interactions of AFPs with ice determine their potential applications in agriculture, food preservation and medicine. AFPs control the shapes of ice crystals in a manner characteristic of the particular AFP type. Moderately active AFPs cause the formation of elongated bipyramidal crystals, often with seemingly defined facets, while hyperactive AFPs produce more varied crystal shapes. These different morphologies are generally considered to be growth shapes. In a series of bright light and fluorescent microscopy observations of ice crystals in solutions containing different AFPs, we show that crystal shaping also occurs during melting. In particular, the characteristic ice shapes observed in solutions of most hyperactive AFPs are formed during melting. We relate these findings to the affinities of the hyperactive AFPs for the basal plane of ice. Our results demonstrate the relation between basal plane affinity and hyperactivity and show a clear difference in the ice-shaping mechanisms of most moderate and hyperactive AFPs. This study provides key aspects associated with the identification of hyperactive AFPs.
Figures
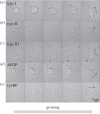

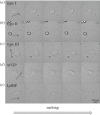




References
-
- Raymond J. A., Fritsen C., Shen K. 2007. An ice-binding protein from an Antarctic sea ice bacterium. FEMS Microbiol. Ecol. 61, 214–221 10.1111/j.1574-6941.2007.00345.x (doi:10.1111/j.1574-6941.2007.00345.x) - DOI - PubMed
-
- Clarke C. J., Buckley S. L., Lindner N. 2002. Ice structuring proteins: a new name for antifreeze proteins. Cryo Lett. 23, 89–92 - PubMed
-
- Fletcher G. L., Hew C. L., Davies P. L. 2001. Antifreeze proteins of teleost fishes. Annu. Rev. Physiol. 63, 359–390 10.1146/annurev.physiol.63.1.359 (doi:10.1146/annurev.physiol.63.1.359) - DOI - PubMed
-
- Duman J. G. 2001. Antifreeze and ice nucleator proteins in terrestrial arthropods. Annu. Rev. Physiol. 63, 327–357 10.1146/annurev.physiol.63.1.327 (doi:10.1146/annurev.physiol.63.1.327) - DOI - PubMed
-
- Yeh Y., Feeney R. E. 1996. Antifreeze proteins: structures and mechanisms of function. Chem. Rev. 96, 601–618 10.1021/cr950260c (doi:10.1021/cr950260c) - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
