Human islet amyloid polypeptide at the air-aqueous interface: a Langmuir monolayer approach
- PMID: 22787008
- PMCID: PMC3479924
- DOI: 10.1098/rsif.2012.0368
Human islet amyloid polypeptide at the air-aqueous interface: a Langmuir monolayer approach
Abstract
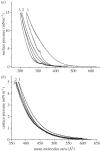
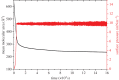
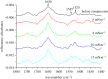
Human islet amyloid polypeptide (hIAPP) is the source of the major component of the amyloid deposits found in the islets of Langerhans of around 95 per cent type 2 diabetic patients. The formation of aggregates and mature fibrils is thought to be responsible for the dysfunction and death of the insulin-producing pancreatic β-cells. Investigation on the conformation, orientation and self-assembly of the hIAPP at time zero could be beneficial for our understanding of its stability and aggregation process. To obtain these insights, the hIAPP at time zero was studied at the air-aqueous interface using the Langmuir monolayer technique. The properties of the hIAPP Langmuir monolayer at the air-aqueous interface on a NaCl subphase with pH 2.0, 5.6 and 9.0 were examined by surface pressure- and potential-area isotherms, UV-Vis absorption, fluorescence spectroscopy and Brewster angle microscopy. The conformational and orientational changes of the hIAPP Langmuir monolayer under different surface pressures were characterized by p-polarized infrared-reflection absorption spectroscopy, and the results did not show any prominent changes of conformation or orientation. The predominant secondary structure of the hIAPP at the air-aqueous interface was α-helix conformation, with a parallel orientation to the interface during compression. These results showed that the hIAPP Langmuir monolayer at the air-aqueous interface was stable, and no aggregate or domain of the hIAPP at the air-aqueous interface was observed during the time of experiments.
Figures





References
-
- Shaw J. E., Sicree R. A., Zimmet P. Z. 2010. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 87, 4–14 10.1016/j.diabres.2009.10.007 (doi:10.1016/j.diabres.2009.10.007) - DOI - PubMed
-
- Song S. H. 2008. Review. Early-onset type 2 diabetes mellitus: a condition with elevated cardiovascular risk? Br. J. Diabetes Vasc. Dis. 8, 61–65 10.1177/14746514080080020201 (doi:10.1177/14746514080080020201) - DOI
-
- Alberti G., Zimmet P., Shaw J., Bloomgarden Z., Kaufman F., Silink M. 2004. Type 2 diabetes in the young: the evolving epidemic. Diabetes Care 27, 1798–1811 10.2337/diacare.27.7.1798 (doi:10.2337/diacare.27.7.1798) - DOI - PubMed
-
- Gedulin B., Cooper G. J. S., Young A. A. 1991. Amylin secretion from the perfused pancreas: Dissociation from insulin and abnormal elevation in insulin-resistant diabetic rats. Biochem. Biophys. Res. Commun. 180, 782–789 10.1016/S0006-291X(05)81133-7 (doi:10.1016/S0006-291X(05)81133-7) - DOI - PubMed
-
- Westermark P., Li Z.-C., Westermark G. T., Leckström A., Steiner D. F. 1996. Effects of beta cell granule components on human islet amyloid polypeptide fibril formation. FEBS Lett. 379, 203–206 10.1016/0014-5793(95)01512-4 (doi:10.1016/0014-5793(95)01512-4) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
