Long-term potentiation in the neonatal rat barrel cortex in vivo
- PMID: 22787036
- PMCID: PMC6622258
- DOI: 10.1523/JNEUROSCI.1212-12.2012
Long-term potentiation in the neonatal rat barrel cortex in vivo
Abstract
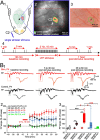
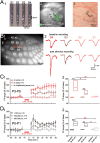
Long-term potentiation (LTP) is important for the activity-dependent formation of early cortical circuits. In the neonatal rodent barrel cortex, LTP has been studied only in vitro. We combined voltage-sensitive dye imaging with extracellular multielectrode recordings to study whisker stimulation-induced LTP in the whisker-to-barrel cortex pathway of the neonatal rat barrel cortex in vivo. Single whisker stimulation at 2 Hz for 10 min induced an age-dependent expression of LTP in postnatal day (P) 0 to P14 rats, with the strongest expression of LTP at P3-P5. The magnitude of LTP was largest in the activated barrel-related column, smaller in the surrounding septal region, and no LTP could be observed in the neighboring barrel. Current source density analyses revealed an LTP-associated increase of synaptic current sinks in layer IV/lower layer II/III at P3-P5 and in the cortical plate/upper layer V at P0-P1. Our study demonstrates for the first time an age-dependent and spatially confined LTP in the barrel cortex of the newborn rat in vivo.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures

References
-
- Barth AL, Malenka RC. NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci. 2001;4:235–236. - PubMed
-
- Berger T, Borgdorff A, Crochet S, Neubauer FB, Lefort S, Fauvet B, Ferezou I, Carleton A, Lüscher HR, Petersen CC. Combined voltage and calcium epifluorescence imaging in vitro and in vivo reveals subthreshold and suprathreshold dynamics of mouse barrel cortex. J Neurophysiol. 2007;97:3751–3762. - PubMed
-
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
