Preparing to reach: selecting an adaptive long-latency feedback controller
- PMID: 22787039
- PMCID: PMC3407880
- DOI: 10.1523/JNEUROSCI.4275-11.2012
Preparing to reach: selecting an adaptive long-latency feedback controller
Abstract
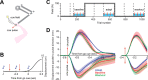
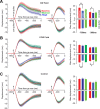
In a voluntary movement, the nervous system specifies not only the motor commands but also the gains associated with reaction to sensory feedback. For example, suppose that, during reaching, a perturbation tends to push the hand to the left. With practice, the brain not only learns to produce commands that predictively compensate for the perturbation but also increases the long-latency reflex gain associated with leftward displacements of the arm. That is, the brain learns a feedback controller. Here, we wondered whether, during the preparatory period before the reach, the brain engaged this feedback controller in anticipation of the upcoming movement. If so, its signature might be present in how the motor system responds to perturbations in the preparatory period. Humans trained on a reach task in which they adapted to a force field. During the preparatory period before the reach, we measured how the arm responded to a pulse to the hand that was either in the direction of the upcoming field, or in the opposite direction. Reach adaptation produced an increase in the long-latency (45-100 ms delay) feedback gains with respect to baseline, but only for perturbations that were in the same direction as the force field that subjects expected to encounter during the reach. Therefore, as the brain prepares for a reach, it loads a feedback controller specific to the upcoming reach. With adaptation, this feedback controller undergoes a change, increasing the gains for the expected sensory feedback.
Figures



References
-
- Bonnet M. Anticipatory changes of long-latency stretch responses during preparation for directional hand movements. Brain Res. 1983;280:51–62. - PubMed
-
- Burdet E, Osu R, Franklin DW, Milner TE, Kawato M. The central nervous system stabilizes unstable dynamics by learning optimal impedance. Nature. 2001;414:446–449. - PubMed
-
- Carlsen AN, Chua R, Timothy Inglis J, Sanderson DJ, Franks IM. Motor preparation in an anticipation-timing task. Exp Brain Res. 2008;190:453–461. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
