Blood-brain barrier permeability is increased after acute adult stroke but not neonatal stroke in the rat
- PMID: 22787045
- PMCID: PMC3539825
- DOI: 10.1523/JNEUROSCI.5977-11.2012
Blood-brain barrier permeability is increased after acute adult stroke but not neonatal stroke in the rat
Abstract
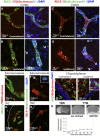
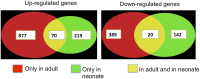
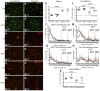
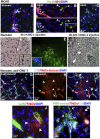
The immaturity of the CNS at birth greatly affects injury after stroke but the contribution of the blood-brain barrier (BBB) to the differential response to stroke in adults and neonates is poorly understood. We asked whether the structure and function of the BBB is disrupted differently in neonatal and adult rats by transient middle cerebral artery occlusion. In adult rats, albumin leakage into injured regions was markedly increased during 2-24 h reperfusion but leakage remained low in the neonates. Functional assays employing intravascular tracers in the neonates showed that BBB permeability to both large (70 kDa dextran) and small (3 kDa dextran), gadolinium (III)-diethyltriaminepentaacetic acid tracers remained largely undisturbed 24 h after reperfusion. The profoundly different functional integrity of the BBB was associated with the largely nonoverlapping patterns of regulated genes in endothelial cells purified from injured and uninjured adult and neonatal brain at 24 h (endothelial transcriptome, 31,042 total probe sets). Within significantly regulated 1266 probe sets in injured adults and 361 probe sets in neonates, changes in the gene expression of the basal lamina components, adhesion molecules, the tight junction protein occludin, and matrix metalloproteinase-9 were among the key differences. The protein expression of collagen-IV, laminin, claudin-5, occludin, and zonula occludens protein 1 was also better preserved in neonatal rats. Neutrophil infiltration remained low in acutely injured neonates but neutralization of cytokine-induced neutrophil chemoattractant-1 in the systemic circulation enhanced neutrophil infiltration, BBB permeability, and injury. The markedly more integrant BBB in neonatal brain than in adult brain after acute stroke may have major implications for the treatment of neonatal stroke.
Figures



References
-
- Afshar-Kharghan V, Thiagarajan P. Leukocyte adhesion and thrombosis. Curr Opin Hematol. 2006;13:34–39. - PubMed
-
- Anthony DC, Bolton SJ, Fearn S, Perry VH. Age-related effects of interleukin-1 beta on polymorphonuclear neutrophil-dependent increases in blood-brain barrier permeability in rats. Brain. 1997;120(Pt 3):435–444. - PubMed
-
- Anthony D, Dempster R, Fearn S, Clements J, Wells G, Perry VH, Walker K. CXC chemokines generate age-related increases in neutrophil-mediated brain inflammation and blood-brain barrier breakdown. Curr Biol. 1998;8:923–926. - PubMed
-
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. - PubMed
-
- Barone FC, Hillegass LM, Price WJ, White RF, Lee EV, Feuerstein GZ, Sarau HM, Clark RK, Griswold DE. Polymorphonuclear leukocyte infiltration into cerebral focal ischemic tissue: myeloperoxidase activity assay and histologic verification. J Neurosci Res. 1991;29:336–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
