Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea
- PMID: 22787049
- PMCID: PMC3417821
- DOI: 10.1523/JNEUROSCI.1064-12.2012
Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea
Abstract
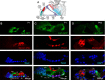
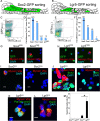
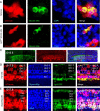
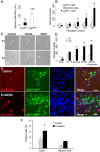
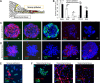
Auditory hair cells are surrounded on their basolateral aspects by supporting cells, and these two cell types together constitute the sensory epithelium of the organ of Corti, which is the hearing apparatus of the ear. We show here that Lgr5, a marker for adult stem cells, was expressed in a subset of supporting cells in the newborn and adult murine cochlea. Lgr5-expressing supporting cells, sorted by flow cytometry and cultured in a single-cell suspension, compared with unsorted cells, displayed an enhanced capacity for self-renewing neurosphere formation in response to Wnt and were converted to hair cells at a higher (>10-fold) rate. The greater differentiation of hair cells in the neurosphere assay showed that Lgr5-positive cells had the capacity to act as cochlear progenitor cells, and lineage tracing confirmed that Lgr5-expressing cells accounted for the cells that formed neurospheres and differentiated to hair cells. The responsiveness to Wnt of cells with a capacity for division and sensory cell formation suggests a potential route to new hair cell generation in the adult cochlea.
Figures

References
-
- Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, Okano H, Sawamoto K. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. - PubMed
-
- Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. 2010;138:1681–1696. - PubMed
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
-
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
