Glycan binding avidity determines the systemic fate of adeno-associated virus type 9
- PMID: 22787229
- PMCID: PMC3457279
- DOI: 10.1128/JVI.01155-12
Glycan binding avidity determines the systemic fate of adeno-associated virus type 9
Abstract
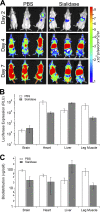
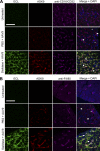
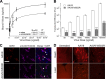
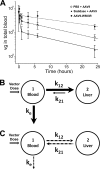
Glycans are key determinants of host range and transmissibility in several pathogens. In the case of adeno-associated viruses (AAV), different carbohydrates serve as cellular receptors in vitro; however, their contributions in vivo are less clear. A particularly interesting example is adeno-associated virus serotype 9 (AAV9), which displays systemic tropism in mice despite low endogenous levels of its primary receptor (galactose) in murine tissues. To understand this further, we studied the effect of modulating glycan binding avidity on the systemic fate of AAV9 in mice. Intravenous administration of recombinant sialidase increased tissue levels of terminally galactosylated glycans in several murine tissues. These conditions altered the systemic tropism of AAV9 into a hepatotropic phenotype, characterized by markedly increased sequestration within the liver sinusoidal endothelium and Kupffer cells. In contrast, an AAV9 mutant with decreased glycan binding avidity displayed a liver-detargeted phenotype. Altering glycan binding avidity also profoundly affected AAV9 persistence in blood circulation. Our results support the notion that high glycan receptor binding avidity appears to impart increased liver tropism, while decreased avidity favors systemic spread of AAV vectors. These findings may not only help predict species-specific differences in tropism for AAV9 on the basis of tissue glycosylation profiles, but also provide a general approach to tailor AAV vectors for systemic or hepatic gene transfer by reengineering capsid-glycan interactions.
Figures


Similar articles
-
Terminal N-linked galactose is the primary receptor for adeno-associated virus 9.J Biol Chem. 2011 Apr 15;286(15):13532-40. doi: 10.1074/jbc.M110.210922. Epub 2011 Feb 17. J Biol Chem. 2011. PMID: 21330365 Free PMC article.
-
Functional analysis of the putative integrin recognition motif on adeno-associated virus 9.J Biol Chem. 2015 Jan 16;290(3):1496-504. doi: 10.1074/jbc.M114.608281. Epub 2014 Nov 17. J Biol Chem. 2015. PMID: 25404742 Free PMC article.
-
Engraftment of a galactose receptor footprint onto adeno-associated viral capsids improves transduction efficiency.J Biol Chem. 2013 Oct 4;288(40):28814-23. doi: 10.1074/jbc.M113.482380. Epub 2013 Aug 12. J Biol Chem. 2013. PMID: 23940044 Free PMC article.
-
Adeno-associated viral vectors and their redirection to cell-type specific receptors.Adv Genet. 2009;67:29-60. doi: 10.1016/S0065-2660(09)67002-4. Adv Genet. 2009. PMID: 19914449 Review.
-
Gene therapy for the CNS using AAVs: The impact of systemic delivery by AAV9.J Control Release. 2016 Nov 10;241:94-109. doi: 10.1016/j.jconrel.2016.09.011. Epub 2016 Sep 13. J Control Release. 2016. PMID: 27637390 Review.
Cited by
-
Innate immune surveillance of the circulation: A review on the removal of circulating virions from the bloodstream.PLoS Pathog. 2022 May 5;18(5):e1010474. doi: 10.1371/journal.ppat.1010474. eCollection 2022 May. PLoS Pathog. 2022. PMID: 35511797 Free PMC article. Review.
-
Natural variations in AAVHSC16 significantly reduce liver tropism and maintain broad distribution to periphery and CNS.Mol Ther Methods Clin Dev. 2022 Jun 30;26:224-238. doi: 10.1016/j.omtm.2022.06.013. eCollection 2022 Sep 8. Mol Ther Methods Clin Dev. 2022. PMID: 35859693 Free PMC article.
-
Genetic dissection of the neuro-glio-vascular machinery in the adult brain.Mol Brain. 2018 Jan 15;11(1):2. doi: 10.1186/s13041-017-0345-4. Mol Brain. 2018. PMID: 29335006 Free PMC article.
-
rAAV9 combined with renal vein injection is optimal for kidney-targeted gene delivery: conclusion of a comparative study.Gene Ther. 2014 Jun;21(6):618-28. doi: 10.1038/gt.2014.35. Epub 2014 May 1. Gene Ther. 2014. PMID: 24784447 Free PMC article.
-
Glycosylation in SARS-CoV-2 variants: A path to infection and recovery.Biochem Pharmacol. 2022 Dec;206:115335. doi: 10.1016/j.bcp.2022.115335. Epub 2022 Oct 31. Biochem Pharmacol. 2022. PMID: 36328134 Free PMC article. Review.
References
-
- Agbandje-McKenna M, Kleinschmidt J. 2011. AAV capsid structure and cell interactions. Methods Mol. Biol. 807:47–92 - PubMed
-
- Altheide TK, et al. 2006. System-wide genomic and biochemical comparisons of sialic acid biology among primates and rodents: evidence for two modes of rapid evolution. J. Biol. Chem. 281:25689–25702 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

