Cost effectiveness of guanfacine extended release as an adjunctive therapy to a stimulant compared with stimulant monotherapy for the treatment of attention-deficit hyperactivity disorder in children and adolescents
- PMID: 22788263
- PMCID: PMC3576910
- DOI: 10.2165/11632920-000000000-00000
Cost effectiveness of guanfacine extended release as an adjunctive therapy to a stimulant compared with stimulant monotherapy for the treatment of attention-deficit hyperactivity disorder in children and adolescents
Abstract
Background: Attention-deficit hyperactivity disorder (ADHD) is a common psychiatric disorder in childhood, affecting 3-7% of school-age children in the US and imposing substantial economic burden. Stimulants are considered first-line pharmacological treatment and are the most prescribed treatment for ADHD. However, approximately 30% of children with ADHD do not have an optimal response to a single stimulant and may require adjunctive therapy.
Objective: Our objective was to conduct a cost-effectiveness analysis (CEA) of adding a non-stimulant, guanfacine extended release (GXR), to stimulants versus maintaining existing stimulant monotherapy in the treatment of ADHD in children and adolescents with suboptimal response to stimulant monotherapy.
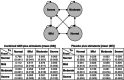
Methods: A 1-year Markov model was developed to estimate costs and effectiveness from a US third-party payer perspective. Effectiveness was measured by the QALY. The model assumed that patients transitioned among four health states (normal, mild, moderate and severe), defined by the Clinical Global Impression-Severity (CGI-S) scale. Transition probabilities were estimated in an ordered logit model using patient-level data from a multicentre, 9-week, double-blind, placebo-controlled, dose-optimization study, where subjects (n = 461) continued their stable morning stimulant and were randomized to GXR administered in the morning, GXR administered in the evening, or placebo. The model assumed that patients in moderate/severe health states after week 8 would discontinue ADHD treatment and remain in that state for the rest of the study period. Direct costs included drug wholesale acquisition costs and health state costs, all in $US, year 2010 values. Utility associated with each health state was obtained from the literature and disutilities associated with adverse events were applied for the first 4 weeks. One-way sensitivity analyses and probabilistic sensitivity analysis (PSA) were conducted by varying costs, utilities, adverse-event duration, and transition probabilities.
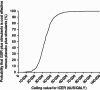
Results: Compared with maintaining existing stimulant monotherapy, adding GXR to existing stimulant monotherapy was associated with an incremental drug cost of $US1016 but a lower medical cost of $US124, resulting in a total incremental cost of $US892 at 1 year. The addition of GXR to stimulants led to an incremental QALY of 0.03 and an incremental cost-effectiveness ratio (ICER) of $US31,660/QALY. In one-way sensitivity analysis, ICER values ranged from $US19,723, when 100% of patients were assumed to be severe in their initial health state, to $US46,631, when the last observed states from the clinical trial were carried forward to the end of the 1-year analysis period. PSA demonstrated a 94.6% likelihood that the ICER falls below $US50,000/QALY.
Conclusions: The impairment associated with residual ADHD symptoms after stimulant therapy is becoming increasingly recognized. This is the first analysis of the cost effectiveness of stimulants combined with an adjunctive medication. This study suggests that the adjunctive therapy of GXR with stimulants is a cost-effective treatment based on a willingness-to-pay threshold of $US50,000/QALY. This may address an unmet need among patients with suboptimal response to stimulant monotherapy.
Figures




Similar articles
-
Is adjunctive pharmacotherapy in attention-deficit/hyperactivity disorder cost-effective in Canada: a cost-effectiveness assessment of guanfacine extended-release as an adjunctive therapy to a long-acting stimulant for the treatment of ADHD.BMC Psychiatry. 2016 Jan 16;16:11. doi: 10.1186/s12888-016-0708-x. BMC Psychiatry. 2016. PMID: 26774811 Free PMC article.
-
Cost effectiveness of guanfacine extended-release versus atomoxetine for the treatment of attention-deficit/hyperactivity disorder: application of a matching-adjusted indirect comparison.Appl Health Econ Health Policy. 2012 Nov 1;10(6):381-95. doi: 10.1007/BF03261873. Appl Health Econ Health Policy. 2012. PMID: 23113551 Clinical Trial.
-
Guanfacine extended release as adjunctive therapy to psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder.Adv Ther. 2012 May;29(5):385-400. doi: 10.1007/s12325-012-0020-1. Epub 2012 May 18. Adv Ther. 2012. PMID: 22610723 Review.
-
Guanfacine extended release adjunctive to a psychostimulant in the treatment of comorbid oppositional symptoms in children and adolescents with attention-deficit/hyperactivity disorder.J Child Adolesc Psychopharmacol. 2014 Jun;24(5):245-52. doi: 10.1089/cap.2013.0103. J Child Adolesc Psychopharmacol. 2014. PMID: 24945085 Free PMC article. Clinical Trial.
-
Evaluation of the current data on guanfacine extended release for the treatment of ADHD in children and adolescents.Expert Opin Pharmacother. 2020 Mar;21(4):417-426. doi: 10.1080/14656566.2019.1706480. Epub 2020 Jan 23. Expert Opin Pharmacother. 2020. PMID: 31971448 Review.
Cited by
-
US-Based Drug Cost Parameter Estimation for Economic Evaluations.Med Decis Making. 2015 Jul;35(5):622-32. doi: 10.1177/0272989X14563987. Epub 2014 Dec 22. Med Decis Making. 2015. PMID: 25532826 Free PMC article.
-
Attention Deficit Hyperactivity Disorder Presentations to The Child and Adolescent Mental Health Urgent Consult Clinic.J Can Acad Child Adolesc Psychiatry. 2019 Aug;28(2):66-71. Epub 2019 Aug 1. J Can Acad Child Adolesc Psychiatry. 2019. PMID: 31447904 Free PMC article.
-
Is adjunctive pharmacotherapy in attention-deficit/hyperactivity disorder cost-effective in Canada: a cost-effectiveness assessment of guanfacine extended-release as an adjunctive therapy to a long-acting stimulant for the treatment of ADHD.BMC Psychiatry. 2016 Jan 16;16:11. doi: 10.1186/s12888-016-0708-x. BMC Psychiatry. 2016. PMID: 26774811 Free PMC article.
-
Profile of guanfacine extended release and its potential in the treatment of attention-deficit hyperactivity disorder.Neuropsychiatr Dis Treat. 2015 May 28;11:1359-70. doi: 10.2147/NDT.S65735. eCollection 2015. Neuropsychiatr Dis Treat. 2015. PMID: 26064054 Free PMC article. Review.
-
The estimation of utility weights in cost-utility analysis for mental disorders: a systematic review.Pharmacoeconomics. 2013 Dec;31(12):1131-54. doi: 10.1007/s40273-013-0107-9. Pharmacoeconomics. 2013. PMID: 24293216
References
-
- Attention deficit hyperactivity disorder: diagnosis and management of ADHD in children, young people and adults [NICE clinical guideline no. 72] Leicester: British Psychological Society; 2009. - PubMed
-
- Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: American Psychiatric Association; 2000.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

