Synergistic and Selective Cancer Cell Killing Mediated by the Oncolytic Adenoviral Mutant AdΔΔ and Dietary Phytochemicals in Prostate Cancer Models
- PMID: 22788991
- PMCID: PMC3440020
- DOI: 10.1089/hum.2012.046
Synergistic and Selective Cancer Cell Killing Mediated by the Oncolytic Adenoviral Mutant AdΔΔ and Dietary Phytochemicals in Prostate Cancer Models
Abstract
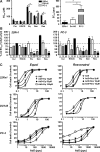
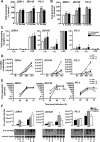
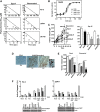
AdΔΔ is an oncolytic adenoviral mutant that has been engineered to selectively target tumors with deregulated cell cycle and apoptosis pathways. AdΔΔ potentiates apoptotic cell death induced by drugs, including mitoxantrone and docetaxel, which are commonly used to treat prostate cancer. Here, we demonstrate that AdΔΔ can also interact synergistically with dietary phytochemicals known to have anti-cancer activities, without incurring the toxic side effects of chemodrugs. Curcumin, genistein, epigallocatechin-gallate, equol, and resveratrol efficiently killed both androgen-receptor positive (22Rv1) and negative cell lines (PC-3, DU145) in combination with adenoviral mutants. Synergistic cell killing was demonstrated with wild-type virus (Ad5) and AdΔΔ in combination with equol and resveratrol. EC(50) values for both phytochemicals and viruses were reduced three- to eightfold in all three combination-treated cell lines. The most potent efficacy was achieved in the cytotoxic drug- and virus-insensitive PC-3 cells, both in vitro and in vivo, while cell killing in normal bronchial epithelial cells was not enhanced. Although equol and resveratrol induced only low levels of apoptosis when administered alone, in combination with wild-type virus or AdΔΔ, the level of apoptotic cell death was significantly increased in PC-3 and DU145 cells. In vivo studies using suboptimal doses of AdΔΔ and equol or resveratrol, showed reduced tumor growth without toxicity to normal tissue. These findings identify novel functions for AdΔΔ and phytochemicals in promoting cancer cell killing and apoptosis, suggesting the use of these natural nontoxic compounds might be a feasible and currently unexploited anti-cancer strategy.
Figures


References
-
- Akaza H. Miyanaga N. Takashima N., et al. Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn. J. Clin. Oncol. 2004;34:86–89. - PubMed
-
- Anders M. Christian C. McMahon M., et al. Inhibition of the Raf/MEK/ERK pathway up-regulates expression of the coxsackievirus and adenovirus receptor in cancer cells. Cancer Res. 2003;63:2088–2095. - PubMed
-
- Arico S. Petiot A. Bauvy C., et al. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J. Biol. Chem. 2001;276:35243–35246. - PubMed
-
- Bewley M.C. Springer K. Zhang Y.B., et al. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science. 1999;286:1579–1583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

