Identification of chronic stress-activated regions reveals a potential recruited circuit in rat brain
- PMID: 22789020
- PMCID: PMC4538599
- DOI: 10.1111/j.1460-9568.2012.08161.x
Identification of chronic stress-activated regions reveals a potential recruited circuit in rat brain
Abstract
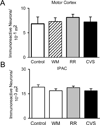
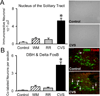
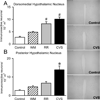
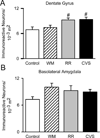
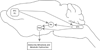
Chronic stress induces presynaptic and postsynaptic modifications in the paraventricular nucleus of the hypothalamus that are consistent with enhanced excitatory hypothalamo-pituitary-adrenocortical (HPA) axis drive. The brain regions mediating these molecular modifications are not known. We hypothesized that chronic variable stress (CVS) tonically activates stress-excitatory regions that interact with the paraventricular nucleus of the hypothalamus, culminating in stress facilitation. In order to identify chronically activated brain regions, ΔFosB, a documented marker of tonic neuronal activation, was assessed in known stress regulatory limbic and brainstem sites. Four experimental groups were included: CVS, repeated restraint (RR) (control for HPA habituation), animals weight-matched (WM) to CVS animals (control for changes in circulating metabolic factors due to reduced weight gain), and non-handled controls. CVS, (but not RR or WM) induced adrenal hypertrophy, indicating that sustained HPA axis drive only occurred in the CVS group. CVS (but not RR or WM) selectively increased the number of FosB/ΔFosB nuclei in the nucleus of the solitary tract, posterior hypothalamic nucleus, and both the infralimbic and prelimbic divisions of the medial prefrontal cortex, indicating an involvement of these regions in chronic drive of the HPA axis. Increases in FosB/ΔFosB-immunoreactive cells were observed following both RR and CVS in the other regions (e.g. the dorsomedial hypothalamus), suggesting activation by both habituating and non-habituating stress conditions. The data suggest that unpredictable stress uniquely activates interconnected cortical, hypothalamic, and brainstem nuclei, potentially revealing the existence of a recruited circuitry mediating chronic drive of brain stress effector systems.
© 2012 The Authors. European Journal of Neuroscience © 2012 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Figures


References
-
- Abrahamson EE, Moore RY. The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res. 2001;889(1–2):1–22. - PubMed
-
- Akana SF, Dallman MF, Bradbury MJ, Scribner KA, Strack AM, Walker CD. Feedback and facilitation in the adrenocortical system: unmasking facilitation by partial inhibition of the glucocorticoid response to prior stress. Endocrinology. 1992;131(1):57–68. - PubMed
-
- Bailey TW, Dimicco JA. Chemical stimulation of the dorsomedial hypothalamus elevates plasma ACTH in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2001;280(1):R8–R15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

