Effect of vaccine dose on the safety and immunogenicity of a candidate TB vaccine, MVA85A, in BCG vaccinated UK adults
- PMID: 22789508
- PMCID: PMC3424417
- DOI: 10.1016/j.vaccine.2012.06.084
Effect of vaccine dose on the safety and immunogenicity of a candidate TB vaccine, MVA85A, in BCG vaccinated UK adults
Abstract
Purpose: A non-randomised, open-label, Phase I safety and immunogenicity dose-finding study to assess the safety and immunogenicity of the candidate TB vaccine Modified Vaccinia virus Ankara expressing Antigen 85A (MVA85A) from Mycobacterium tuberculosis (MTB) in healthy adult volunteers previously vaccinated with BCG.
Methods: Healthy BCG-vaccinated volunteers were vaccinated with either 1×10(7) or 1×10(8)PFU of MVA85A. All adverse events were documented and antigen specific T cell responses were measured using an ex vivo IFN-γ ELISPOT assay. Safety and immunogenicity were compared between the 2 dose groups and with a previous trial in which a dose of 5×10(7)PFU MVA85A had been administered.
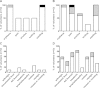
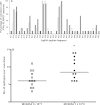
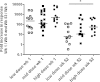
Results: There were no serious adverse events recorded following administration of either 1×10(7) or 1×10(8)PFU of MVA85A. Systemic adverse events were more frequently reported following administration of 1×10(8)PFU of MVA85A when compared to either 5×10(7) or 1×10(7)PFU of MVA85A but were mild or moderate in severity and resolved completely within 7 days of immunisation. Antigen specific T cell responses as measured by the IFN-γ ELISPOT were significantly higher following immunisation in adults receiving 1×10(8)PFU compared to the 5×10(7) and 1×10(7) doses. Additionally, a broader range of Ag85A epitopes are detected following 1×10(8)PFU of MVA85A.
Conclusion: A higher dose of 1×10(8)PFU of MVA85A is well-tolerated, increases the frequency of IFN-γ secreting T cells detected following immunisation and broadens the range of Ag85A epitopes detected.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
MVA85A vaccine to enhance BCG for preventing tuberculosis.Cochrane Database Syst Rev. 2019 Apr 30;4(4):CD012915. doi: 10.1002/14651858.CD012915.pub2. Cochrane Database Syst Rev. 2019. PMID: 31038197 Free PMC article.
-
Safety and immunogenicity of an FP9-vectored candidate tuberculosis vaccine (FP85A), alone and with candidate vaccine MVA85A in BCG-vaccinated healthy adults: a phase I clinical trial.Hum Vaccin Immunother. 2013 Jan;9(1):50-62. doi: 10.4161/hv.22464. Epub 2012 Nov 10. Hum Vaccin Immunother. 2013. PMID: 23143773 Free PMC article. Clinical Trial.
-
Comparing the safety and immunogenicity of a candidate TB vaccine MVA85A administered by intramuscular and intradermal delivery.Vaccine. 2013 Feb 4;31(7):1026-33. doi: 10.1016/j.vaccine.2012.12.042. Epub 2012 Dec 21. Vaccine. 2013. PMID: 23266342 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of a candidate tuberculosis vaccine MVA85A delivered by aerosol in BCG-vaccinated healthy adults: a phase 1, double-blind, randomised controlled trial.Lancet Infect Dis. 2014 Oct;14(10):939-46. doi: 10.1016/S1473-3099(14)70845-X. Epub 2014 Aug 20. Lancet Infect Dis. 2014. PMID: 25151225 Free PMC article. Clinical Trial.
-
The Systematic Review and Meta-Analysis on the Immunogenicity and Safety of the Tuberculosis Subunit Vaccines M72/AS01E and MVA85A.Front Immunol. 2020 Oct 8;11:1806. doi: 10.3389/fimmu.2020.01806. eCollection 2020. Front Immunol. 2020. PMID: 33133057 Free PMC article.
Cited by
-
Phase I Trial Evaluating the Safety and Immunogenicity of Candidate TB Vaccine MVA85A, Delivered by Aerosol to Healthy M.tb-Infected Adults.Vaccines (Basel). 2021 Apr 16;9(4):396. doi: 10.3390/vaccines9040396. Vaccines (Basel). 2021. PMID: 33923628 Free PMC article.
-
MVA85A vaccine to enhance BCG for preventing tuberculosis.Cochrane Database Syst Rev. 2019 Apr 30;4(4):CD012915. doi: 10.1002/14651858.CD012915.pub2. Cochrane Database Syst Rev. 2019. PMID: 31038197 Free PMC article.
-
A subunit vaccine Ag85A-LpqH focusing on humoral immunity provides substantial protection against tuberculosis in mice.iScience. 2024 Dec 20;28(1):111568. doi: 10.1016/j.isci.2024.111568. eCollection 2025 Jan 17. iScience. 2024. PMID: 39868033 Free PMC article.
-
Novel Modified Vaccinia Virus Ankara Vector Expressing Anti-apoptotic Gene B13R Delays Apoptosis and Enhances Humoral Responses.J Virol. 2019 Feb 19;93(5):e01648-18. doi: 10.1128/JVI.01648-18. Print 2019 Mar 1. J Virol. 2019. PMID: 30541829 Free PMC article.
-
Lessons learnt from the first efficacy trial of a new infant tuberculosis vaccine since BCG.Tuberculosis (Edinb). 2013 Mar;93(2):143-9. doi: 10.1016/j.tube.2013.01.003. Epub 2013 Feb 12. Tuberculosis (Edinb). 2013. PMID: 23410889 Free PMC article. Clinical Trial.
References
-
- Trunz B.B., Fine P., Dye C. Effect of BCG vaccination on childhood Tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. - PubMed
-
- Scriba T.J., Tameris M., Mansoor N., Smit E., van der Merwe L. Dose-finding study of the novel tuberculosis vaccine, MVA85A, in healthy BCG-vaccinated infants. J Infect Dis. 2011;203:1832–1843. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

