The changing face of HIV vaccine research
- PMID: 22789610
- PMCID: PMC3499796
- DOI: 10.7448/IAS.15.2.17407
The changing face of HIV vaccine research
Abstract
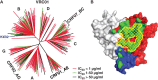
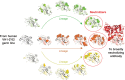
While there has been remarkable progress in understanding the biology of HIV-1 and its recognition by the human immune system, we have not yet developed an efficacious HIV-1 vaccine. Vaccine challenges include the genetic diversity and mutability of HIV-1 which create a plethora of constantly changing antigens, the structural features of the viral envelope glycoprotein that disguise conserved receptor-binding sites from the immune system, and the presence of carbohydrate moieties that shield potential epitopes from antibodies. Despite these challenges, there has been significant scientific progress in recent years. In 2009, a large-scale clinical trial known as RV144 demonstrated that a HIV-1 vaccine could modestly reduce the incidence of HIV-1 infection. Further, the identification of broadly neutralizing monoclonal antibodies (such as VRC01, a human monoclonal antibody capable of neutralizing over 90% of natural HIV-1 isolates, as well as PG and PGT antibodies that recognize conserved glycopeptide epitopes) has revealed new opportunities for vaccine design. Our ability to understand HIV-1 structure and antibody epitopes at the atomic level, the rapid advance of computational and bioinformatics approaches to immunogen design, and our newly acquired knowledge that it is possible for a vaccine to reduce the risk of HIV-1 infection, have all opened up new and promising pathways towards the development of an urgently needed effective HIV-1 vaccine. This article summarizes challenges to the development of an HIV-1 vaccine, lessons learned from scientific investigation and completed vaccine trials, and promising developments in HIV-1 vaccine design.
Figures
Similar articles
-
A Trimeric HIV-1 Envelope gp120 Immunogen Induces Potent and Broad Anti-V1V2 Loop Antibodies against HIV-1 in Rabbits and Rhesus Macaques.J Virol. 2018 Feb 12;92(5):e01796-17. doi: 10.1128/JVI.01796-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237847 Free PMC article.
-
Features of Recently Transmitted HIV-1 Clade C Viruses that Impact Antibody Recognition: Implications for Active and Passive Immunization.PLoS Pathog. 2016 Jul 19;12(7):e1005742. doi: 10.1371/journal.ppat.1005742. eCollection 2016 Jul. PLoS Pathog. 2016. PMID: 27434311 Free PMC article.
-
Advances in HIV-1 Vaccine Development.Viruses. 2018 Apr 1;10(4):167. doi: 10.3390/v10040167. Viruses. 2018. PMID: 29614779 Free PMC article. Review.
-
The Hard Way towards an Antibody-Based HIV-1 Env Vaccine: Lessons from Other Viruses.Viruses. 2018 Apr 15;10(4):197. doi: 10.3390/v10040197. Viruses. 2018. PMID: 29662026 Free PMC article. Review.
-
Proteins mimicking epitope of HIV-1 virus neutralizing antibody induce virus-neutralizing sera in mice.EBioMedicine. 2019 Sep;47:247-256. doi: 10.1016/j.ebiom.2019.07.015. EBioMedicine. 2019. PMID: 31544770 Free PMC article.
Cited by
-
Generation of cellular immune memory and B-cell immunity is impaired by natural killer cells.Nat Commun. 2015 Feb 27;6:6375. doi: 10.1038/ncomms7375. Nat Commun. 2015. PMID: 25721802 Free PMC article.
-
Neutralizing antibodies to HIV-1 induced by immunization.J Exp Med. 2013 Feb 11;210(2):209-23. doi: 10.1084/jem.20121827. J Exp Med. 2013. PMID: 23401570 Free PMC article. Review.
-
Willingness to participate in HIV therapeutic vaccine trials among HIV-infected patients on ART in China.PLoS One. 2014 Nov 5;9(11):e111321. doi: 10.1371/journal.pone.0111321. eCollection 2014. PLoS One. 2014. PMID: 25372044 Free PMC article.
-
Expanding possibilities for intervention against small ruminant lentiviruses through genetic marker-assisted selective breeding.Viruses. 2013 Jun 14;5(6):1466-99. doi: 10.3390/v5061466. Viruses. 2013. PMID: 23771240 Free PMC article. Review.
-
Comprehensive epitope mapping using polyclonally expanded human CD8 T cells and a two-step ELISpot assay for testing large peptide libraries.J Immunol Methods. 2021 Apr;491:112970. doi: 10.1016/j.jim.2021.112970. Epub 2021 Jan 30. J Immunol Methods. 2021. PMID: 33529681 Free PMC article.
References
-
- Starcich BR, Hahn BH, Shaw GM, McNeely PD, Modrow S, Wolf H, et al. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-WLAV, the retrovirus of AIDS. Cell. 1986;45:637–46. - PubMed
-
- Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, et al. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393(6686):705–11. - PubMed
-
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, et al. Antibody neutralization and escape by HIV. Nature. 2003;422(6929):307–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

