A hydrogel derived from decellularized dermal extracellular matrix
- PMID: 22789723
- PMCID: PMC3408574
- DOI: 10.1016/j.biomaterials.2012.06.051
A hydrogel derived from decellularized dermal extracellular matrix
Abstract
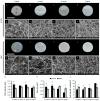
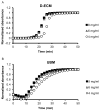
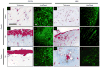
The ECM of mammalian tissues has been used as a scaffold to facilitate the repair and reconstruction of numerous tissues. Such scaffolds are prepared in many forms including sheets, powders, and hydrogels. ECM hydrogels provide advantages such as injectability, the ability to fill an irregularly shaped space, and the inherent bioactivity of native matrix. However, material properties of ECM hydrogels and the effect of these properties upon cell behavior are neither well understood nor controlled. The objective of this study was to prepare and determine the structure, mechanics, and the cell response in vitro and in vivo of ECM hydrogels prepared from decellularized porcine dermis and urinary bladder tissues. Dermal ECM hydrogels were characterized by a more dense fiber architecture and greater mechanical integrity than urinary bladder ECM hydrogels, and showed a dose dependent increase in mechanical properties with ECM concentration. In vitro, dermal ECM hydrogels supported greater C2C12 myoblast fusion, and less fibroblast infiltration and less fibroblast mediated hydrogel contraction than urinary bladder ECM hydrogels. Both hydrogels were rapidly infiltrated by host cells, primarily macrophages, when implanted in a rat abdominal wall defect. Both ECM hydrogels degraded by 35 days in vivo, but UBM hydrogels degraded more quickly, and with greater amounts of myogenesis than dermal ECM. These results show that ECM hydrogel properties can be varied and partially controlled by the scaffold tissue source, and that these properties can markedly affect cell behavior.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures





References
-
- Van Vlierberghe S, Dubruel P, Schacht E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules. 2011;12(5):1387–408. - PubMed
-
- Freytes DO, Martin J, Velankar SS, Lee AS, Badylak SF. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 2008;29(11):1630–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

