Myocardial infarction and intramyocardial injection models in swine
- PMID: 22790084
- PMCID: PMC3936356
- DOI: 10.1038/nprot.2012.075
Myocardial infarction and intramyocardial injection models in swine
Abstract
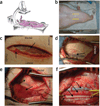
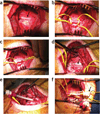
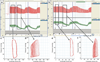
Sustainable and reproducible large animal models that closely replicate the clinical sequelae of myocardial infarction (MI) are important for the translation of basic science research into bedside medicine. Swine are well accepted by the scientific community for cardiovascular research, and they represent an established animal model for preclinical trials for US Food and Drug Administration (FDA) approval of novel therapies. Here we present a protocol for using porcine models of MI created with a closed-chest coronary artery occlusion-reperfusion technique. This creates a model of MI encompassing the anteroapical, lateral and septal walls of the left ventricle. This model infarction can be easily adapted to suit individual study design and enables the investigation of a variety of possible interventions. This model is therefore a useful tool for translational research into the pathophysiology of ventricular remodeling and is an ideal testing platform for novel biological approaches targeting regenerative medicine. This model can be created in approximately 8-10 h.
Figures




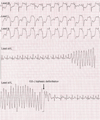
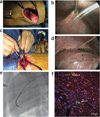

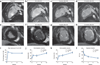
References
-
- Heidenreich PA, et al. Forecasting the future of cardiovascular disease in the United States. Circulation. 2011;123:933–944. - PubMed
-
- Swindle M. Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques. Taylor & Francis Group; 2007.
-
- Dondelinger RF, et al. Relevant radiological anatomy of the pig as a training model in interventional radiology. Eur. Radiol. 1998;8:1254–1273. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

