Ubiquitylation of active Rac1 by the E3 ubiquitin-ligase HACE1
- PMID: 22790197
- PMCID: PMC3408975
- DOI: 10.4161/sgtp.19221
Ubiquitylation of active Rac1 by the E3 ubiquitin-ligase HACE1
Abstract
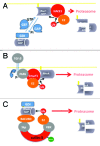
Rho GTPases undergo ubiquitylation and degradation via the ubiquitin-proteasome pathway. We now report in the November issue of Developmental Cell that the E3 ubiquitin-ligase HACE1 catalyzes the ubiquitylation of GTP-bound Rac1. Depletion of HACE1 leads to an increase of Rac1 activity. We have proposed that HACE1 limits Rac1 activity in cells, a regulation that is usurped by some pathogenic bacteria for efficient invasion of host cell monolayers. We here review these findings in parallel with the regulation of RhoA by the ubiquitin and proteasome system (UPS) and discuss the impact of these regulations on the capacity of Rho GTPases to signal.
Figures
Comment on
- Torrino S, Visvikis O, Doye A, Boyer L, Stefani C, Munro P, Bertoglio J, Gacon G, Mettouchi A, Lemichez E. The E3 ubiquitin-ligase HACE1 catalyzes the ubiquitylation of active Rac1. Dev Cell. 2011;21:959–65. doi: 10.1016/j.devcel.2011.08.015. doi: 10.1016/j.devcel.2011.08.015
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
