Systematic analysis of hydroxyethyl starch (HES) reviews: proliferation of low-quality reviews overwhelms the results of well-performed meta-analyses
- PMID: 22790311
- PMCID: PMC3783958
- DOI: 10.1007/s00134-012-2614-0
Systematic analysis of hydroxyethyl starch (HES) reviews: proliferation of low-quality reviews overwhelms the results of well-performed meta-analyses
Abstract
Purpose: Hydroxyethyl starch (HES) is a synthetic colloid used widely for resuscitation despite the availability of safer, less costly fluids. Numerous HES reviews have been published that may have influenced clinicians' practice. We have therefore examined the relationship between the methodological quality of published HES reviews, authors' potential conflicts of interest (pCOI) and the recommendations made.
Methods: Systematic analysis of reviews on HES use.
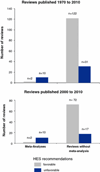
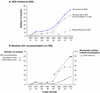
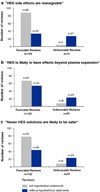
Results: Between 1975 and 2010, 165 reviews were published containing recommendations for or against HES use. From the 1990s onwards, favorable reviews increased from two to eight per year and HES's share of the artificial colloid market tripled from 20 to 60 %. Only 7 % (12/165) of these reviews of HES use contained meta-analyses; these 7 % had higher Overview Quality Assessment Questionnaire (OQAQ) scores [median (range) 6.5 (3-7)] than reviews without meta-analysis [2 (1-4); p < 0.001]. The rates of recommending against HES use are 83 % (10/12) in meta-analyses and 20 % (31/153) in reviews without meta-analysis (p < 0.0001). Fourteen authors published the majority (70/124) of positive reviews, and ten of these 14 had or have since developed a pCOI with various manufacturers of HES.
Conclusions: Low-quality HES reviews reached different conclusions than high-quality meta-analyses from independent entities, such as Cochrane Reviews. The majority of these low-quality positive HES reviews were written by a small group of authors, most of whom had or have since established ties to industry. The proliferation of positive HES reviews has been associated with increased utilization of an expensive therapy despite the lack of evidence for meaningful clinical benefit and increased risks. Clinicians need to be more informed that marketing efforts are potentially influencing scientific literature.
Conflict of interest statement
Conflicts of interest
C. Hartog, H. Skupin, J. Sun, and C. Natanson declare that they have no conflict of interest. K. Reinhart has in the past received an unrestricted grant for the conduct of the VISEP study and speaker’s and consultancy fees from B. Braun, Melsungen, Germany. B.Braun, Melsungen also contributed to the German Sepsis Society to fund an endowed professorship for clinical sepsis research at the University Hospital of Jena.
Figures


Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Starch safety in resuscitation - when will we ever learn?S Afr Med J. 2013 Apr 29;103(6):365-7. doi: 10.7196/samj.6969. S Afr Med J. 2013. PMID: 23725952 Review.
-
Fluid therapy in critical illness: a special focus on indication, the use of hydroxyethyl starch and its different raw materials.Curr Opin Anaesthesiol. 2013 Jun;26(3):253-60. doi: 10.1097/ACO.0b013e3283606b71. Curr Opin Anaesthesiol. 2013. PMID: 23492982 Review.
-
Small volume resuscitation with 7.5% hypertonic saline, hydroxyethyl starch 130/0.4 solution and hypertonic sodium chloride hydroxyethyl starch 40 injection reduced lung injury in endotoxin shock rats: comparison with saline.Pulm Pharmacol Ther. 2012 Feb;25(1):27-32. doi: 10.1016/j.pupt.2011.10.003. Epub 2011 Oct 24. Pulm Pharmacol Ther. 2012. PMID: 22037283
-
Hydroxyethyl starches in equine medicine.J Vet Emerg Crit Care (San Antonio). 2019 Jul;29(4):349-359. doi: 10.1111/vec.12854. Epub 2019 Jun 22. J Vet Emerg Crit Care (San Antonio). 2019. PMID: 31228334 Review.
Cited by
-
Resuscitation with polymeric plasma substitutes is permissive for systemic inflammatory response syndrome and sepsis in multiply injured patients: a retrospective cohort study.Eur J Med Res. 2016 Oct 13;21(1):39. doi: 10.1186/s40001-016-0227-8. Eur J Med Res. 2016. PMID: 27737718 Free PMC article.
-
The Mass Production of Redundant, Misleading, and Conflicted Systematic Reviews and Meta-analyses.Milbank Q. 2016 Sep;94(3):485-514. doi: 10.1111/1468-0009.12210. Milbank Q. 2016. PMID: 27620683 Free PMC article.
-
Concerns over use of hydroxyethyl starch solutions.BMJ. 2014 Nov 10;349:g5981. doi: 10.1136/bmj.g5981. BMJ. 2014. PMID: 25385352 Free PMC article.
-
Financial conflicts of interest in systematic reviews: associations with results, conclusions, and methodological quality.Cochrane Database Syst Rev. 2019 Aug 5;8(8):MR000047. doi: 10.1002/14651858.MR000047.pub2. Cochrane Database Syst Rev. 2019. PMID: 31425611 Free PMC article.
-
Reporting bias in trials of volume resuscitation with hydroxyethyl starch.Wien Klin Wochenschr. 2014 Apr;126(7-8):189-94. doi: 10.1007/s00508-014-0503-y. Epub 2014 Mar 5. Wien Klin Wochenschr. 2014. PMID: 24596076 Free PMC article. Review.
References
-
- Schortgen F, Deye N, Brochard L. Preferred plasma volume expanders for critically ill patients: results of an international survey. Intensive Care Med. 2004;30:2222–2229. - PubMed
-
- The FLUIDS study investigators for the Scandinavian Critical Care Trials Group. Preferences for colloid use in Scandinavian intensive care units. Acta Anaesthesiol Scand. 2008;52:750–758. - PubMed
-
- Basora M, Moral V, Llau JV, Silva S. Perioperative colloid administration: a survey of Spanish anesthesiologists’ attitudes. Rev Esp Anestesiol Reanim. 2007;54:162–168. - PubMed
-
- Liu FC, Liao CH, Chang YW, Liou JT, Day YJ. Hydroxyethyl starch interferes with human blood ex vivo coagulation, platelet function and sedimentation. Acta Anaesthesiol Taiwan. 2009;47:71–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

