Heat stress induces epithelial plasticity and cell migration independent of heat shock factor 1
- PMID: 22791010
- PMCID: PMC3468677
- DOI: 10.1007/s12192-012-0349-z
Heat stress induces epithelial plasticity and cell migration independent of heat shock factor 1
Abstract
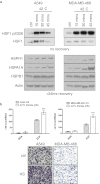
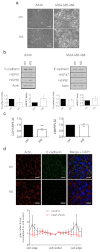
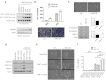
Current cancer therapies including cytotoxic chemotherapy, radiation and hyperthermic therapy induce acute proteotoxic stress in tumour cells. A major challenge to cancer therapeutic efficacy is the recurrence of therapy-resistant tumours and how to overcome their emergence. The current study examines the concept that tumour cell exposure to acute proteotoxic stress results in the acquisition of a more advanced and aggressive cancer cell phenotype. Specifically, we determined whether heat stress resulted in an epithelial-to-mesenchymal transition (EMT) and/or the enhancement of cell migration, components of an advanced and therapeutically resistant cancer phenotype. We identified that heat stress enhanced cell migration in both the lung A549, and breast MDA-MB-468 human adenocarcinoma cell lines, with A549 cells also undergoing a partial EMT. Moreover, in an in vivo model of thermally ablated liver metastases of the mouse colorectal MoCR cell line, immunohistological analysis of classical EMT markers demonstrated a shift to a more mesenchymal phenotype in the surviving tumour fraction, further demonstrating that thermal stress can induce epithelial plasticity. To identify a mechanism by which thermal stress modulates epithelial plasticity, we examined whether the major transcriptional regulator of the heat shock response, heat shock factor 1 (HSF1), was a required component. Knockdown of HSF1 in the A549 model did not prevent the associated morphological changes or enhanced migratory profile of heat stressed cells. Therefore, this study provides evidence that heat stress significantly impacts upon cancer cell epithelial plasticity and the migratory phenotype independent of HSF1. These findings further our understanding of novel biological downstream effects of heat stress and their potential independence from the classical heat shock pathway.
Figures


Similar articles
-
Heterogeneous nuclear ribonucleoprotein K inhibits heat shock-induced transcriptional activity of heat shock factor 1.J Biol Chem. 2017 Aug 4;292(31):12801-12812. doi: 10.1074/jbc.M117.774992. Epub 2017 Jun 7. J Biol Chem. 2017. PMID: 28592492 Free PMC article.
-
HSF1 promotes the inhibition of EMT-associated migration by low glucose via directly regulating Snail1 expression in HCC cells.Discov Med. 2016 Sep;22(120):87-96. Discov Med. 2016. PMID: 27755964
-
The maximal cytoprotective function of the heat shock protein 27 is dependent on heat shock protein 70.Biochim Biophys Acta. 2011 Jan;1813(1):129-35. doi: 10.1016/j.bbamcr.2010.08.012. Epub 2010 Oct 8. Biochim Biophys Acta. 2011. PMID: 20934464 Free PMC article.
-
Glioma progression through the prism of heat shock protein mediated extracellular matrix remodeling and epithelial to mesenchymal transition.Exp Cell Res. 2017 Oct 15;359(2):299-311. doi: 10.1016/j.yexcr.2017.08.032. Epub 2017 Aug 26. Exp Cell Res. 2017. PMID: 28844885 Review.
-
Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: an update.Arch Toxicol. 2013 Jan;87(1):19-48. doi: 10.1007/s00204-012-0918-z. Epub 2012 Aug 11. Arch Toxicol. 2013. PMID: 22885793 Free PMC article. Review.
Cited by
-
Intestinal Barrier Integrity in Heat-Stressed Modern Broilers and Their Ancestor Wild Jungle Fowl.Front Vet Sci. 2020 May 7;7:249. doi: 10.3389/fvets.2020.00249. eCollection 2020. Front Vet Sci. 2020. PMID: 32457922 Free PMC article.
-
The Helicobacter pylori cytotoxin CagA is essential for suppressing host heat shock protein expression.Cell Stress Chaperones. 2016 May;21(3):523-33. doi: 10.1007/s12192-016-0680-x. Epub 2016 Mar 1. Cell Stress Chaperones. 2016. PMID: 26928021 Free PMC article.
-
Expression of the Alternative Oxidase Influences Jun N-Terminal Kinase Signaling and Cell Migration.Mol Cell Biol. 2018 Nov 28;38(24):e00110-18. doi: 10.1128/MCB.00110-18. Print 2018 Dec 15. Mol Cell Biol. 2018. PMID: 30224521 Free PMC article.
-
Regulation of a Novel Splice Variant of Early Growth Response 4 (EGR4-S) by HER+ Signalling and HSF1 in Breast Cancer.Cancers (Basel). 2022 Mar 18;14(6):1567. doi: 10.3390/cancers14061567. Cancers (Basel). 2022. PMID: 35326716 Free PMC article.
-
Enhancement of Farnesoid X Receptor Inhibits Migration, Adhesion and Angiogenesis through Proteasome Degradation and VEGF Reduction in Bladder Cancers.Int J Mol Sci. 2022 May 9;23(9):5259. doi: 10.3390/ijms23095259. Int J Mol Sci. 2022. PMID: 35563650 Free PMC article.
References
-
- Baritaki S, Chapman A, Yeung K, Spandidos DA, Palladino M, Bonavida B. Inhibition of epithelial to mesenchymal transition in metastatic prostate cancer cells by the novel proteasome inhibitor, NPI-0052: pivotal roles of snail repression and RKIP induction. Oncogene. 2009;28(40):3573–3585. doi: 10.1038/onc.2009.214. - DOI - PubMed
-
- Cannito S, Novo E, Compagnone A, Valfre di Bonzo L, Busletta C, Zmara E, Paternostro C, Povero D, Bandino A, Bozzo F, Cravanzola C, Bravoco V, Colombatto S, Parola M. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis. 2008;29(12):2267–2278. doi: 10.1093/carcin/bgn216. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

