Dendritic spines: from structure to in vivo function
- PMID: 22791026
- PMCID: PMC3410382
- DOI: 10.1038/embor.2012.102
Dendritic spines: from structure to in vivo function
Abstract
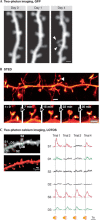
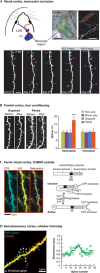
Dendritic spines arise as small protrusions from the dendritic shaft of various types of neuron and receive inputs from excitatory axons. Ever since dendritic spines were first described in the nineteenth century, questions about their function have spawned many hypotheses. In this review, we introduce understanding of the structural and biochemical properties of dendritic spines with emphasis on components studied with imaging methods. We then explore advances in in vivo imaging methods that are allowing spine activity to be studied in living tissue, from super-resolution techniques to calcium imaging. Finally, we review studies on spine structure and function in vivo. These new results shed light on the development, integration properties and plasticity of spines.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- García-López P, García-Marín V, Freire M (2007) The discovery of dendritic spines by Cajal in 1888 and its relevance in the present neuroscience. Prog Neurobiol 83: 110–130 - PubMed
-
- Shepherd GM (1996) The dendritic spine: a multifunctional integrative unit. J Neurophysiol 75: 2197–2210 - PubMed
-
- Peters A, Kaiserman-Abramof IR (1970) The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am J Anat 127: 321–355 - PubMed
-
- Jones EG, Powell TP (1969) Morphological variations in the dendritic spines of the neocortex. J Cell Sci 5: 509–529 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

