SOCS3 is an endogenous inhibitor of pathologic angiogenesis
- PMID: 22791286
- PMCID: PMC3466973
- DOI: 10.1182/blood-2012-04-422527
SOCS3 is an endogenous inhibitor of pathologic angiogenesis
Erratum in
-
Stahl A, Joyal J-S, Chen J, et al. SOCS3 is an endogenous inhibitor of pathologic angiogenesis. Blood. 2012;120(14):2925-2929.Blood. 2018 Dec 13;132(24):2614. doi: 10.1182/blood-2018-10-880906. Blood. 2018. PMID: 30545895 Free PMC article. No abstract available.
Abstract
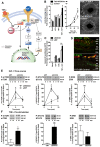
Inflammatory cytokines and growth factors drive angiogenesis independently; however, their integrated role in pathologic and physiologic angiogenesis is not fully understood. Suppressor of cytokine signaling-3 (SOCS3) is an inducible negative feedback regulator of inflammation and growth factor signaling. In the present study, we show that SOCS3 curbs pathologic angiogenesis. Using a Cre/Lox system, we deleted SOCS3 in vessels and studied developmental and pathologic angiogenesis in murine models of oxygen-induced retinopathy and cancer. Conditional loss of SOCS3 leads to increased pathologic neovascularization, resulting in pronounced retinopathy and increased tumor size. In contrast, physiologic vascularization is not regulated by SOCS3. In vitro, SOCS3 knockdown increases proliferation and sprouting of endothelial cells costimulated with IGF-1 and TNFα via reduced feedback inhibition of the STAT3 and mTOR pathways. These results identify SOCS3 as a pivotal endogenous feedback inhibitor of pathologic angiogenesis and a potential therapeutic target acting at the converging crossroads of growth factor- and cytokine-induced vessel growth.
Figures

References
-
- Wang J, Campbell IL. Cytokine signaling in the brain: putting a SOCS in it? J Neurosci Res. 2002;67(4):423–427. - PubMed
-
- Starr R, Willson TA, Viney EM, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387(6636):917–921. - PubMed
-
- Lebel E, Vallieres L, Rivest S. Selective involvement of interleukin-6 in the transcriptional activation of the suppressor of cytokine signaling-3 in the brain during systemic immune challenges. Endocrinology. 2000;141(10):3749–3763. - PubMed
-
- Dey BR, Furlanetto RW, Nissley P. Suppressor of cytokine signaling (SOCS)-3 protein interacts with the insulin-like growth factor-I receptor. Biochem Biophys Res Commun. 2000;278(1):38–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

