Inactivation of heparan sulfate 2-O-sulfotransferase accentuates neutrophil infiltration during acute inflammation in mice
- PMID: 22791291
- PMCID: PMC3429312
- DOI: 10.1182/blood-2012-03-417139
Inactivation of heparan sulfate 2-O-sulfotransferase accentuates neutrophil infiltration during acute inflammation in mice
Abstract
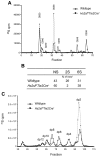
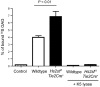
Neutrophil recruitment and extravasation at sites of inflammation provide a mechanism for host defense. We showed previously that heparan sulfate, a type of sulfated glycosaminoglycan, facilitates neutrophil recruitment based on the reduction of neutrophil infiltration in mice in which the overall sulfation of the chains was reduced by selective inactivation of N-acetylglucosamine N-deacetylase-N-sulfotransferase (Ndst1) in endothelial cells. Here we show that inactivation of uronyl 2-O-sulfotransferase in endothelial cells (Hs2st), an enzyme that acts downstream from Ndst1, results in enhanced neutrophil recruitment in several models of acute inflammation. Enhanced neutrophil infiltration resulted in part from reduced rolling velocity under flow both in vivo and in vitro, which correlated with stronger binding of neutrophil L-selectin to mutant endothelial cells. Hs2st-deficient endothelial cells also displayed a striking increase in binding of IL-8 and macrophage inflammatory protein-2. The enhanced binding of these mediators of neutrophil recruitment resulted from a change in heparan sulfate structure caused by increased N-sulfation and 6-O-sulfation of glucosamine units in response to the decrease in 2-O-sulfation of uronic acid residues. This gain-of-function phenotype provides formidable evidence demonstrating the importance of endothelial heparan sulfate in inflammation and suggests a novel enzyme target for enhancing the innate immune response.
Figures




References
-
- Ley K, Cerrito M, Arfors KE. Sulfated polysaccharides inhibit leukocyte rolling in rabbit mesentery venules. Am J Physiol. 1991;260:1667–1673. - PubMed
-
- Young E. The anti-inflammatory effects of heparin and related compounds. Thromb Res. 2008;122(6):743–752. - PubMed
-
- Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol. 2005;6(9):902–910. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

